The Effect of Xevinapant Combined with Ionizing Radiation on HNSCC and Normal Tissue Cells and the Impact of Xevinapant on Its Targeted Proteins cIAP1 and XIAP
- PMID: 37371123
- PMCID: PMC10297233
- DOI: 10.3390/cells12121653
The Effect of Xevinapant Combined with Ionizing Radiation on HNSCC and Normal Tissue Cells and the Impact of Xevinapant on Its Targeted Proteins cIAP1 and XIAP
Abstract
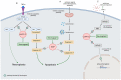
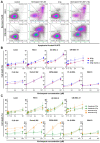
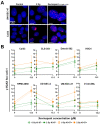
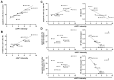
The poor prognosis of HNSCC is partly due to treatment resistance. The SMAC mimetic Xevinapant is a promising new approach to targeted cancer therapy. Xevinapant inhibits cIAP1/2 and XIAP, leading to apoptosis, necroptosis and inhibition of prosurvival signaling. Combining Xevinapant with IR could improve therapeutic potential. The effect of Xevinapant in combination with IR on HNSCC and healthy tissue cells was investigated. Cell growth, cell death, clonogenic survival and DNA double-strand breaks (DSBs) were studied, and intracellular cIAP1 and XIAP levels were evaluated. Xevinapant had cytostatic and cytotoxic, as well as radiosensitizing, effects on the malignant cells, while healthy tissue cells were less affected. Apoptotic and necrotic cell death was particularly affected, but the increase in residual DSBs and the reduced survival implied an additional effect of Xevinapant on DNA damage repair and other cell inactivation mechanisms. cIAP1 and XIAP levels varied for each cell line and were affected by Xevinapant and IR treatment. There was an association between higher IAP levels and increased cell death. Xevinapant appears to be a potent new drug for HNSCC therapy, especially in combination with IR. IAP levels could be an indicator for impaired DNA damage repair and increased susceptibility to cellular stress.
Keywords: XIAP; Xevinapant; apoptosis; cIAP1/2; head and neck squamous cell carcinoma; inhibitor; interactions; ionizing radiation; targeted therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
LCL161, a SMAC-mimetic, Preferentially Radiosensitizes Human Papillomavirus-negative Head and Neck Squamous Cell Carcinoma.Mol Cancer Ther. 2019 Jun;18(6):1025-1035. doi: 10.1158/1535-7163.MCT-18-1157. Epub 2019 Apr 23. Mol Cancer Ther. 2019. PMID: 31015310 Free PMC article.
-
Inhibiting the inhibitors: Development of the IAP inhibitor xevinapant for the treatment of locally advanced squamous cell carcinoma of the head and neck.Cancer Treat Rev. 2023 Feb;113:102492. doi: 10.1016/j.ctrv.2022.102492. Epub 2022 Nov 30. Cancer Treat Rev. 2023. PMID: 36640618 Free PMC article. Review.
-
Dual Antagonist of cIAP/XIAP ASTX660 Sensitizes HPV- and HPV+ Head and Neck Cancers to TNFα, TRAIL, and Radiation Therapy.Clin Cancer Res. 2019 Nov 1;25(21):6463-6474. doi: 10.1158/1078-0432.CCR-18-3802. Epub 2019 Jul 2. Clin Cancer Res. 2019. PMID: 31266830 Free PMC article.
-
ASTX660, a Novel Non-peptidomimetic Antagonist of cIAP1/2 and XIAP, Potently Induces TNFα-Dependent Apoptosis in Cancer Cell Lines and Inhibits Tumor Growth.Mol Cancer Ther. 2018 Jul;17(7):1381-1391. doi: 10.1158/1535-7163.MCT-17-0848. Epub 2018 Apr 25. Mol Cancer Ther. 2018. PMID: 29695633
-
The expanding role of IAP antagonists for the treatment of head and neck cancer.Cancer Med. 2023 Jul;12(13):13958-13965. doi: 10.1002/cam4.6011. Epub 2023 May 2. Cancer Med. 2023. PMID: 37132167 Free PMC article. Review.
Cited by
-
Radioresistance or/and radiosensitivity of head and neck squamous cell carcinoma: biological angle.Oral Maxillofac Surg. 2024 Jun;28(2):547-555. doi: 10.1007/s10006-023-01189-9. Epub 2023 Nov 8. Oral Maxillofac Surg. 2024. PMID: 37935817 Review.
-
Multifaceted Evaluation of Inhibitors of Anti-Apoptotic Proteins in Head and Neck Cancer: Insights from In Vitro, In Vivo, and Clinical Studies (Review).Pharmaceuticals (Basel). 2024 Sep 30;17(10):1308. doi: 10.3390/ph17101308. Pharmaceuticals (Basel). 2024. PMID: 39458950 Free PMC article. Review.
References
-
- Hashibe M., Brennan P., Benhamou S., Castellsague X., Chen C., Curado M.P., Dal Maso L., Daudt A.W., Fabianova E., Fernandez L., et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J. Natl. Cancer Inst. 2007;99:777–789. doi: 10.1093/jnci/djk179. - DOI - PubMed
-
- Blot W.J., McLaughlin J.K., Winn D.M., Austin D.F., Greenberg R.S., Preston-Martin S., Bernstein L., Schoenberg J.B., Stemhagen A., Fraumeni J.F., Jr. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–3287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

