Hybrid Palliation for Hypoplastic Left Heart Syndrome: Role of Echocardiography
- PMID: 37371244
- PMCID: PMC10297217
- DOI: 10.3390/children10061012
Hybrid Palliation for Hypoplastic Left Heart Syndrome: Role of Echocardiography
Abstract
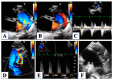
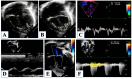
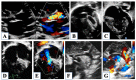
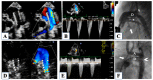
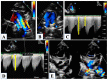
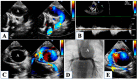
Hypoplastic left heart syndrome is a spectrum of complex congenital cardiac defects. Although in borderline cases, biventricular repair is a viable option, in the majority of cases, univentricular palliation is the treatment of choice. Hybrid palliation can be a valid alternative to classic Norwood operation in the neonatal period, especially in selected cases such as high-risk patients or borderline left ventricles. Echocardiography is the main diagnostic modality in this pediatric population, from the fetal diagnosis to the subsequent surgical steps of palliative treatment. Hybrid palliation is performed after birth and is characterized by surgical banding of the pulmonary arteries along with transcatheter stenting of the ductus arteriosus. There are some peculiar aspects of cardiac imaging that characterize this type of palliation, and that should be considered in the different phases before and after the procedure. We aimed to review the current literature about the role of echocardiography in the management of patients with hypoplastic left heart undergoing hybrid palliation.
Keywords: echocardiography; hybrid palliation; hypoplastic left heart syndrome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Galantowicz M., Cheatham J.P., Phillips A., Cua C.L., Hoffman T.M., Hill S.L., Rodeman R. Hybrid approach for hypoplastic left heart syndrome: Intermediate results after the learning curve. Ann. Thorac. Surg. 2008;85:2063–2070; discussion 2070–2061. doi: 10.1016/j.athoracsur.2008.02.009. - DOI - PubMed
-
- Ceneri N.M., Desai M.H., Tongut A., Ozturk M., Ramakrishnan K., Staffa S.J., Zurakowski D., Donofrio M.T., Downing T., d’Udekem Y., et al. Hybrid strategy in neonates with ductal-dependent systemic circulation and multiple risk factors. J. Thorac. Cardiovasc. Surg. 2022;164:1291–1303 e1296. doi: 10.1016/j.jtcvs.2021.11.103. - DOI - PubMed
-
- Michelfelder E., Gomez C., Border W., Gottliebson W., Franklin C. Predictive value of fetal pulmonary venous flow patterns in identifying the need for atrial septoplasty in the newborn with hypoplastic left ventricle. Circulation. 2005;112:2974–2979. doi: 10.1161/CIRCULATIONAHA.105.534180. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

