CCR4 Blockade Diminishes Intratumoral Macrophage Recruitment and Augments Survival of Syngeneic Pancreatic Cancer-Bearing Mice
- PMID: 37371612
- PMCID: PMC10295441
- DOI: 10.3390/biomedicines11061517
CCR4 Blockade Diminishes Intratumoral Macrophage Recruitment and Augments Survival of Syngeneic Pancreatic Cancer-Bearing Mice
Abstract
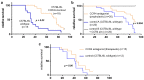
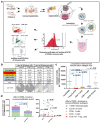
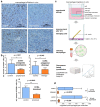
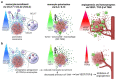
Pancreatic cancer is known for its tumor microenvironment (TME), which is rich in stromal and immune cells supporting cancer growth and therapy resistance. In particular, tumor-associated macrophages (TAMs) are known for their angiogenesis- and metastasis-promoting properties, which lead to the failure of conventional therapies for pancreatic cancer. Hence, treatment options targeting TAMs are needed. The C-C chemokine receptor type 4 (CCR4) is critical for immune cell recruitment into the TME, and in this paper we explore the effects of its genetic or immunotherapeutic blockade in pancreatic-cancer-bearing mice. Murine PDA6606 pancreatic cancer cells and murine peritoneal macrophages were used for in vitro migration assays. In vivo, a syngeneic, orthotropic pancreatic cancer model was established. Tumor growth and survival were monitored under prophylactic and therapeutic application of a CCR4 antagonist (AF-399/420/18025) in wildtype (CCR4wt) and CCR4-knockout (CCR4-/-) mice. Immune infiltration was monitored in tumor tissue sections and via flow cytometry of lysed tumors. PDA6606 cells induced less migration in CCR4-/- than in CCR4wt macrophages in vitro. Pancreatic TAM infiltration was higher, and survival was reduced in CCR4wt mice compared to CCR4-/- mice. Antagonizing CCR4 in wildtype mice revealed similar results as in CCR4-/- mice without antagonization. Prophylactic CCR4 antagonist application in wildtype mice was more efficient than therapeutic antagonization. CCR4 seems to be critically involved in TAM generation and tumor progression in pancreatic cancer. CCR4 blockade may help prolong the relapse-free period after curative surgery in pancreatic cancer and improve prognosis.
Keywords: CCL17; CCL22; M2 macrophages; TAMs; migration; tumor-associated macrophages.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Morimoto Y., Bian Y., Gao P., Yashiro-Ohtani Y., Zhou X.Y., Ono S., Nakahara H., Kogo M., Hamaoka T., Fujiwara H. Induction of Surface Ccr4 and Its Functionality in Mouse Th2 Cells Is Regulated Differently During Th2 Development. J. Leukoc. Biol. 2005;78:753–761. doi: 10.1189/jlb.0305139. - DOI - PubMed
-
- Wang Z., Louras N.J., Lellouch A.G., Pratts S.G., Zhang H., Wang H., Huang C.A., Cetrulo C.L., Jr., Madsen J.C., Sachs D.H., et al. Dosing Optimization of Ccr4 Immunotoxin for Improved Depletion of Ccr4(+) Treg in Nonhuman Primates. Mol. Oncol. 2018;12:1374–1382. doi: 10.1002/1878-0261.12331. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

