Interaction of Drug-Sensitive and -Resistant Human Melanoma Cells with HUVEC Cells: A Label-Free Cell-Based Impedance Study
- PMID: 37371639
- PMCID: PMC10294930
- DOI: 10.3390/biomedicines11061544
Interaction of Drug-Sensitive and -Resistant Human Melanoma Cells with HUVEC Cells: A Label-Free Cell-Based Impedance Study
Abstract
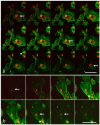
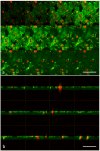
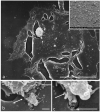
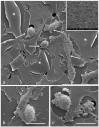
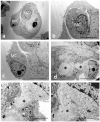
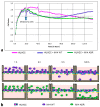
Cancer cell extravasation is a crucial step in cancer metastasis. However, many of the mechanisms involved in this process are only now being elucidated. Thus, in the present study we analysed the trans-endothelial invasion of melanoma cells by a high throughput label-free cell impedance assay applied to transwell chamber invasion assay. This technique monitors and quantifies in real-time the invasion of endothelial cells by malignant tumour cells, for a long time, avoiding artefacts due to preparation of the end point measurements. Results obtained by impedance analysis were compared with endpoint measurements. In this study, we used human melanoma M14 wild type (WT) cells and their drug resistant counterparts, M14 multidrug resistant (ADR) melanoma cells, selected by prolonged exposure to doxorubicin (DOX). Tumour cells were co-cultured with monolayers of human umbilical vein endothelial cells (HUVEC). Results herein reported demonstrated that: (i) the trans-endothelial migration of resistant melanoma cells was faster than sensitive ones; (ii) the endothelial cells appeared to be strongly affected by the transmigration of melanoma cells which showed the ability to degrade their cytoplasm; (iii) resistant cells preferentially adopted the transcellular invasion vs. the paracellular one; (iv) the endothelial damage mediated by tumour metalloproteinases seemed to be reversible.
Keywords: cancer cell extravasation; human melanoma cells; label-free cell impedance assay; light and electron microscopy; multidrug resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Liotta L.A., Rao C.N., Barsky S.H. Tumor invasion and the extracellular matrix. Lab. Investig. 1983;49:636–649. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

