Circulating Angiogenic Factors and Ischemic Diabetic Foot Syndrome Advancement-A Pilot Study
- PMID: 37371653
- PMCID: PMC10295465
- DOI: 10.3390/biomedicines11061559
Circulating Angiogenic Factors and Ischemic Diabetic Foot Syndrome Advancement-A Pilot Study
Abstract
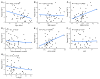
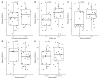
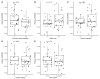
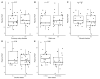
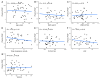
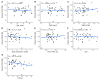
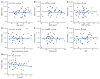
Despite clear evidence of inadequate angiogenesis in ischemic diabetic foot syndrome (DFS) pathogenesis, angiogenic factor level changes in patients with ischemic DFS remain inconsistent. This study aimed to assess circulating angiogenic factors concerning ischemic DFS advancement and describe their relationships with patients' clinical characteristics, microvascular parameters, and diabetic control. The study included 41 patients with ischemic DFS (67.3 (8.84) years; 82.9% males). Angiogenic processes were assessed by identifying circulating concentrations of five pro- and two anti-angiogenic factors. We found that penetrating ulcers were related to a significantly higher FGF-2 level (8.86 (5.29) vs. 5.23 (4.17) pg/mL, p = 0.02). Moreover, plasma FGF-2 showed a significant correlation with the SINBAD score (r = 0.32, p = 0.04), platelet count (r = 0.43, p < 0.01), white cell count (r = 0.42, p < 0.01), and age (r = -0.35, p = 0.03). We did not observe any significant linear relationship between the studied biomarkers and microcirculatory parameters, nor for glycemic control. In a univariate analysis using logistic regression, an increase in plasma FGF-2 was tied to greater odds of high-grade ulcers (OR 1.16; 95% CI 1.02-1.38, p = 0.043). This suggests that circulating FGF-2 may serve as a potential biomarker for predicting DFU advancement and progression. It is necessary to conduct further studies with follow-up observations to confirm this hypothesis.
Keywords: angiogenesis; angiogenic factors; diabetic foot syndrome; microcirculation; peripheral arterial disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
Grants and funding
LinkOut - more resources
Full Text Sources

