Mechanisms of Action and Limitations of Monoclonal Antibodies and Single Chain Fragment Variable (scFv) in the Treatment of Cancer
- PMID: 37371712
- PMCID: PMC10295864
- DOI: 10.3390/biomedicines11061610
Mechanisms of Action and Limitations of Monoclonal Antibodies and Single Chain Fragment Variable (scFv) in the Treatment of Cancer
Abstract
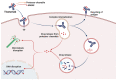
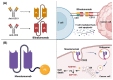
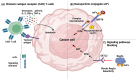
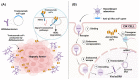
Monoclonal antibodies are among the most effective tools for detecting tumor-associated antigens. The U.S. Food and Drug Administration (FDA) has approved more than 36 therapeutic antibodies for developing novel alternative therapies that have significant success rates in fighting cancer. However, some functional limitations have been described, such as their access to solid tumors and low interaction with the immune system. Single-chain variable fragments (scFv) are versatile and easy to produce, and being an attractive tool for use in immunotherapy models. The small size of scFv can be advantageous for treatment due to its short half-life and other characteristics related to the structural and functional aspects of the antibodies. Therefore, the main objective of this review was to describe the current situation regarding the mechanisms of action, applications, and limitations of monoclonal antibodies and scFv in the treatment of cancer.
Keywords: cancer; mechanism of action; monoclonal antibodies; scFv; treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Álvarez-Vallina L. Anticuerpos Monoclonales. Realidades y Perspectivas. Editorial Complutense; Madrid, Spain: 2004.
Publication types
LinkOut - more resources
Full Text Sources

