PRISMA Systematic Literature Review, including with Meta-Analysis vs. Chatbot/GPT (AI) regarding Current Scientific Data on the Main Effects of the Calf Blood Deproteinized Hemoderivative Medicine (Actovegin) in Ischemic Stroke
- PMID: 37371718
- PMCID: PMC10295843
- DOI: 10.3390/biomedicines11061623
PRISMA Systematic Literature Review, including with Meta-Analysis vs. Chatbot/GPT (AI) regarding Current Scientific Data on the Main Effects of the Calf Blood Deproteinized Hemoderivative Medicine (Actovegin) in Ischemic Stroke
Abstract
Background: Stroke is a significant public health problem and a leading cause of death and long-term disability worldwide. Several treatments for ischemic stroke have been developed, but these treatments have limited effectiveness. One potential treatment for this condition is Actovegin®/AODEJIN, a calf blood deproteinized hemodialysate/ultrafiltrate that has been shown to have pleiotropic/multifactorial and possibly multimodal effects. The actual actions of this medicine are thought to be mediated by its ability to reduce oxidative stress, inflammation, and apoptosis and to enhance neuronal survival and plasticity.
Methods: To obtain the most up-to-date information on the effects of Actovegin®/AODEJIN in ischemic stroke, we systematically reviewed the literature published in the last two years. This review builds upon our previous systematic literature review published in 2020, which used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) method to search for and select related articles over almost two decades, between 1 January 2001 and 31 December 2019. Additionally, we compared the results of our PRISMA search (human intelligence-based) with those obtained from an interrogation of a GPT-based chatbot (ChatGPT) in order to ensure comprehensive coverage of potentially relevant studies.
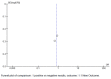
Results: Our updated review found limited new evidence on the use of Actovegin®/AODEJIN in ischemic stroke, although the number of articles on this subject consistently increased compared to that from our initial systematic literature review. Specifically, we found five articles up to 2020 and eight more until December 2022. While these studies suggest that Actovegin®/AODEJIN may have neuroprotective effects in ischemic stroke, further clinical trials are needed to confirm these findings. Consequently, we performed a funnel analysis to evaluate the potential for publication bias.
Discussion: Our funnel analysis showed no evidence of publication bias, suggesting that the limited number of studies identified was not due to publication bias but rather due to a lack of research in this area. However, there are limitations when using ChatGPT, particularly in distinguishing between truth and falsehood and determining the appropriateness of interpolation. Nevertheless, AI can provide valuable support in conducting PRISMA-type systematic literature reviews, including meta-analyses.
Conclusions: The limited number of studies identified in our review highlights the need for additional research in this area, especially as no available therapeutic agents are capable of curing central nervous system lesions. Any contribution, including that of Actovegin (with consideration of a positive balance between benefits and risks), is worthy of further study and periodic reappraisal. The evolving advancements in AI may play a role in the near future.
Keywords: PRISMA-type systematic literature review; chatbot/GPT (artificial intelligence—AI); deproteinized ultrafiltrate/hemodialysate compound Actovegin; ischemic stroke; meta-analysis.
Conflict of interest statement
The authors confirm no conflict of interest. The producers of Actovegin® (Nycomed/Takeda) are constant partners of our societies (the Romanian Society for NeuroRehabilitation, the Romanian Spinal Cord Society, and the Romanian Society of Physical and Rehabilitation Medicine & Balneoclimatology) and Clinic Division. They did not interfere with the study process (data collection or processing, concluding results, or editing endeavors).
Figures


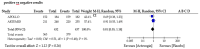
References
-
- Mu Q., Xue Y., Lu Y., Zhang Y., Cheng Q., Wan J., Liu P., Liu J., Qu Y., Huang C., et al. Advances in the therapy of cerebral ischemia-reperfusion injury with natural product-based nanoparticles. Nano TransMed. 2022;1:e9130009. doi: 10.26599/NTM.2022.9130009. - DOI
-
- Onose G., Anghelescu A., Blendea D., Ciobanu V., Daia C., Firan F.C., Oprea M., Spinu A., Popescu C., Ionescu A., et al. Cellular and Molecular Targets for Non-Invasive, Non-Pharmacological Therapeutic/Rehabilitative Interventions in Acute Ischemic Stroke. Int. J. Mol. Sci. 2022;23:907. doi: 10.3390/ijms23020907. - DOI - PMC - PubMed
-
- Huang H., Young W., Ziad A., Hooshang S., Alok S., Dafin M., Shiqing F., Lin C. Beijing Declaration of International Association of Neurorestoratology. J. Neurorestoratol. 2015;3:121–122. doi: 10.2147/JN.S89682. - DOI
-
- Kleindorfer D.O., Towfighi A., Chaturvedi S., Cockroft K.M., Gutierrez J., Lombardi-Hill D., Kamel H., Kernan W.N., Kittner S.J., Leira E.C., et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2021;52:e364–e467. doi: 10.1161/STR.0000000000000375. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

