Cold Physical Plasma-Mediated Fenretinide Prodrug Activation Confers Additive Cytotoxicity in Epithelial Cells
- PMID: 37372001
- PMCID: PMC10295284
- DOI: 10.3390/antiox12061271
Cold Physical Plasma-Mediated Fenretinide Prodrug Activation Confers Additive Cytotoxicity in Epithelial Cells
Abstract
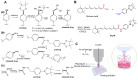
Cold physical plasma is a partially ionized gas operated at body temperature and utilized for heat-sensitive technical and medical purposes. Physical plasma is a multi-component system consisting of, e.g., reactive species, ions and electrons, electric fields, and UV light. Therefore, cold plasma technology is an interesting tool for introducing biomolecule oxidative modifications. This concept can be extended to anticancer drugs, including prodrugs, which could be activated in situ to enhance local anticancer effects. To this end, we performed a proof-of-concept study on the oxidative prodrug activation of a tailor-made boronic pinacol ester fenretinide treated with the atmospheric pressure argon plasma jet kINPen operated with either argon, argon-hydrogen, or argon-oxygen feed gas. Fenretinide release from the prodrug was triggered via Baeyer-Villiger-type oxidation of the boron-carbon bond based on hydrogen peroxide and peroxynitrite, which were generated by plasma processes and chemical addition using mass spectrometry. Fenretinide activation led to additive cytotoxic effects in three epithelial cell lines in vitro compared to the effects of cold plasma treatment alone regarding metabolic activity reduction and an increase in terminal cell death, suggesting that cold physical plasma-mediated prodrug activation is a new direction for combination cancer treatment studies.
Keywords: boronic pinacol ester; cancer therapy; cold atmospheric pressure plasma; gas plasma technology; prodrug; reactive oxygen species (ROS).
Conflict of interest statement
The authors declare no conflict of interest.
Figures











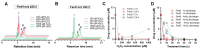


References
Grants and funding
LinkOut - more resources
Full Text Sources

