Each Cellular Compartment Has a Characteristic Protein Reactive Cysteine Ratio Determining Its Sensitivity to Oxidation
- PMID: 37372004
- PMCID: PMC10295259
- DOI: 10.3390/antiox12061274
Each Cellular Compartment Has a Characteristic Protein Reactive Cysteine Ratio Determining Its Sensitivity to Oxidation
Abstract
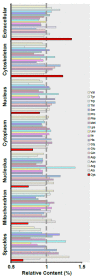
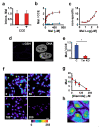
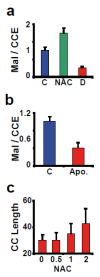
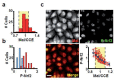
Signaling and detoxification of Reactive Oxygen Species (ROS) are important patho-physiologcal processes. Despite this, we lack comprehensive information on individual cells and cellular structures and functions affected by ROS, which is essential to build quantitative models of the effects of ROS. The thiol groups from cysteines (Cys) in proteins play a major role in redox defense, signaling, and protein function. In this study, we show that the proteins in each subcellular compartment contain a characteristic Cys amount. Using a fluorescent assay for -SH in thiolate form and amino groups in proteins, we show that the thiolate content correlates with ROS sensitivity and signaling properties of each compartment. The highest absolute thiolate concentration was found in the nucleolus, followed by the nucleoplasm and cytoplasm whereas protein thiolate groups per protein showed an inverse pattern. In the nucleoplasm, protein reactive thiols concentrated in SC35 speckles, SMN, and the IBODY that accumulated oxidized RNA. Our findings have important functional consequences, and explain differential sensitivity to ROS.
Keywords: 8-hydroxy guanosine; RNA polymerase; SMN; nuclear speckles; oxidative stress; oxidized RNA; thioredoxin; transcription.
Conflict of interest statement
IC Biomed provided support in the form of salary to author J.C. This does not alter our adherence to all the Scientific Reports policies on sharing data and materials.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

