Derivatives of Differentiation-Inducing Factor 1 Differentially Control Chemotaxis and Stalk Cell Differentiation in Dictyostelium discoideum
- PMID: 37372157
- PMCID: PMC10295651
- DOI: 10.3390/biology12060873
Derivatives of Differentiation-Inducing Factor 1 Differentially Control Chemotaxis and Stalk Cell Differentiation in Dictyostelium discoideum
Abstract
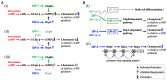
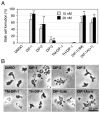
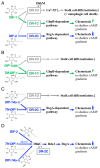
Differentiation-inducing factors 1 and 2 (DIF-1 and DIF-2) are small lipophilic signal molecules that induce stalk cell differentiation but differentially modulate chemotaxis toward cAMP in the cellular slime mold Dictyostelium discoideum; DIF-1 suppresses chemotactic cell movement in shallow cAMP gradients, whereas DIF-2 promotes it. The receptor(s) for DIF-1 and DIF-2 have not yet been identified. We examined the effects of nine derivatives of DIF-1 on chemotactic cell movement toward cAMP and compared their chemotaxis-modulating activity and stalk cell differentiation-inducing activity in wild-type and mutant strains. The DIF derivatives differentially affected chemotaxis and stalk cell differentiation; for example, TM-DIF-1 suppressed chemotaxis and showed poor stalk-inducing activity, DIF-1(3M) suppressed chemotaxis and showed strong stalk-inducing activity, and TH-DIF-1 promoted chemotaxis. These results suggest that DIF-1 and DIF-2 have at least three receptors: one for stalk cell induction and two for chemotaxis modulation. In addition, our results show that the DIF derivatives can be used to analyze the DIF-signaling pathways in D. discoideum.
Keywords: DIF; DhkC; Dictyostelium; GbpB; RdeA; RegA; chemotaxis; stalk cell differentiation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

