Assessing the Integrity of Clinical Trials Included in Evidence Syntheses
- PMID: 37372725
- PMCID: PMC10298200
- DOI: 10.3390/ijerph20126138
Assessing the Integrity of Clinical Trials Included in Evidence Syntheses
Abstract
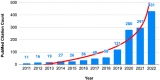
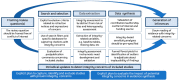
Evidence syntheses of randomized clinical trials (RCTs) offer the highest level of scientific evidence for informing clinical practice and policy. The value of evidence synthesis itself depends on the trustworthiness of the included RCTs. The rising number of retractions and expressions of concern about the authenticity of RCTs has raised awareness about the existence of problematic studies, sometimes called "zombie" trials. Research integrity, i.e., adherence to ethical and professional standards, is a multi-dimensional concept that is incompletely evaluated for the RCTs included in current evidence syntheses. Systematic reviewers tend to rely on the editorial and peer-review system established by journals as custodians of integrity of the RCTs they synthesize. It is now well established that falsified and fabricated RCTs are slipping through. Thus, RCT integrity assessment becomes a necessary step in systematic reviews going forward, in particular because RCTs with data-related integrity concerns remain available for use in evidence syntheses. There is a need for validated tools for systematic reviewers to proactively deploy in the assessment of integrity deviations without having to wait for RCTs to be retracted by journals or expressions of concern issued. This article analyzes the issues and challenges in conducting evidence syntheses where the literature contains RCTs with possible integrity deficits. The way forward in the form of formal RCT integrity assessments in systematic reviews is proposed, and implications of this new initiative are discussed. Future directions include emphasizing ethical and professional standards, providing tailored integrity-specific training, and creating systems to promote research integrity, as improvements in RCT integrity will benefit evidence syntheses.
Keywords: clinical trials; evidence synthesis; research integrity; systematic reviews.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Khan K.S., Zamora J. Systematic Reviews to Support Evidence-Based Medicine. 3rd ed. Taylor & Francis Publishing; London, UK: 2022.
-
- Gopalakrishna G., ter Riet G., Vink G., Stoop I., Wicherts J.M., Bouter L.M. Prevalence of questionable research practices, research misconduct and their potential explanatory factors: A survey among academic researchers in the Netherlands. PLoS ONE. 2022;17:e0263023. doi: 10.1371/journal.pone.0263023. - DOI - PMC - PubMed
-
- Vinkers C.H., Lamberink H.J., Tijdink J.K., Heus P., Bouter L., Glasziou P., Moher D., Damen J.A., Hooft L.O.W. The methodological quality of 176,620 randomized controlled trials published between 1966 and 2018 reveals a positive trend but also an urgent need for improvement. PLoS Biol. 2021;19:e3001162. doi: 10.1371/journal.pbio.3001162. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

