Informed Consent in COVID-19-Research: An Ethical Analysis of Clinical Studies Performed during the Pandemic
- PMID: 37372911
- PMCID: PMC10298309
- DOI: 10.3390/healthcare11121793
Informed Consent in COVID-19-Research: An Ethical Analysis of Clinical Studies Performed during the Pandemic
Abstract
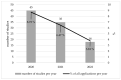
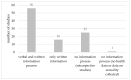
Health crises such as the current COVID-19 pandemic pose challenges to the conduct of clinical studies. Aspects of research ethics, such as obtaining informed consent (IC), can be complicated. We are concerned with whether or not the proper IC procedures were followed in the context of clinical studies at Ulm University in the years 2020 to 2022. We identified all protocols of clinical studies dealing with COVID-19 that the Research Ethics Committee of Ulm University has reviewed and voted on in the years 2020 to 2022. We then performed a thematic analysis regarding the following aspects: study type, handling of IC, type of patient information, means of communication, applied security precautions, and the approach to participants from vulnerable groups. We identified n = 98 studies that dealt with COVID-19. In n = 25 (25.51%), IC was obtained traditionally in written form, in n = 26 (26.53%) IC was waived, in n = 11 (11.22%) IC was obtained delayed, and in n = 19 (19.39%) IC was obtained by proxy. No study protocol was accepted that waived IC in case IC would have been required in times outside of pandemics. It is possible to obtain IC even in times of severe health crises. In the future, it is necessary to address in greater detail and with legal certainty which alternative methods of obtaining IC are possible and under which circumstances IC can be waived.
Keywords: COVID-19; clinical study; ethics; informed consent.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Medical Research during the COVID-19 Pandemic. [(accessed on 14 April 2023)]; Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7441262/
-
- Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on Clinical Trials on Medicinal Products for Human Use, and Repealing Directive 2001/20/EC. [(accessed on 13 April 2023)]. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014R0536.
-
- WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. [(accessed on 8 April 2023)]. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-pr...
-
- Medicinal Products Act (Arzneimittelgesetz—AMG) [(accessed on 15 April 2023)]. Available online: https://www.gesetze-im-internet.de/englisch_amg/index.html.
-
- De Vries J., Burgess T., Blockman M., Ntusi N.A.B. Research on COVID-19 in South Africa: Guiding principles for informed consent. S. Afr. Med. J. 2020;110:635–639. - PubMed
LinkOut - more resources
Full Text Sources