Natural Compound Boldine Lessens Myotonic Dystrophy Type 1 Phenotypes in DM1 Drosophila Models, Patient-Derived Cell Lines, and HSALR Mice
- PMID: 37372969
- PMCID: PMC10298378
- DOI: 10.3390/ijms24129820
Natural Compound Boldine Lessens Myotonic Dystrophy Type 1 Phenotypes in DM1 Drosophila Models, Patient-Derived Cell Lines, and HSALR Mice
Abstract
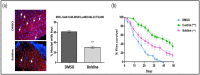
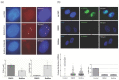
Myotonic dystrophy type 1 (DM1) is a complex rare disorder characterized by progressive muscle dysfunction, involving weakness, myotonia, and wasting, but also exhibiting additional clinical signs in multiple organs and systems. Central dysregulation, caused by an expansion of a CTG trinucleotide repeat in the DMPK gene's 3' UTR, has led to exploring various therapeutic approaches in recent years, a few of which are currently under clinical trial. However, no effective disease-modifying treatments are available yet. In this study, we demonstrate that treatments with boldine, a natural alkaloid identified in a large-scale Drosophila-based pharmacological screening, was able to modify disease phenotypes in several DM1 models. The most significant effects include consistent reduction in nuclear RNA foci, a dynamic molecular hallmark of the disease, and noteworthy anti-myotonic activity. These results position boldine as an attractive new candidate for therapy development in DM1.
Keywords: Drosophila; boldine; drug development; myotonic dystrophy; natural small molecule; patient-derived cells; rare disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Johnson N.E., Butterfield R.J., Mayne K., Newcomb T., Imburgia C., Dunn D., Duval B., Feldkamp M.L., Weiss R.B. Population-Based Prevalence of Myotonic Dystrophy Type 1 Using Genetic Analysis of Statewide Blood Screening Program. Neurology. 2021;96:e1045–e1053. doi: 10.1212/WNL.0000000000011425. - DOI - PMC - PubMed
-
- Harper P.S. In: Myotonic Dystrophy. 3rd ed. Saunders W.B., editor. OUP Oxford; Oxford, UK: 2001.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

