Clinical Trials with Mesenchymal Stem Cell Therapies for Osteoarthritis: Challenges in the Regeneration of Articular Cartilage
- PMID: 37373096
- PMCID: PMC10298392
- DOI: 10.3390/ijms24129939
Clinical Trials with Mesenchymal Stem Cell Therapies for Osteoarthritis: Challenges in the Regeneration of Articular Cartilage
Abstract
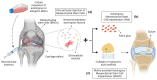
Osteoarthritis (OA) is a whole-joint disease primarily characterized by the deterioration of hyaline cartilage. Current treatments include microfracture and chondrocyte implantation as early surgical strategies that can be combined with scaffolds to repair osteochondral lesions; however, intra-articular (IA) injections or implantations of mesenchymal stem cells (MSCs) are new approaches that have presented encouraging therapeutic results in animal models and humans. We critically reviewed clinical trials with MSC therapies for OA, focusing on their effectiveness, quality, and outcomes in the regeneration of articular cartilage. Several sources of autologous or allogeneic MSCs were used in the clinical trials. Minor adverse events were generally reported, indicating that IA applications of MSCs are potentially safe. The evaluation of articular cartilage regeneration in human clinical trials is challenging, particularly in the inflammatory environment of osteoarthritic joints. Our findings indicate that IA injections of MSCs are efficacious in the treatment of OA and the regeneration of cartilage, but that they may be insufficient for the full repair of articular cartilage defects. The possible interference of clinical and quality variables in the outcomes suggests that robust clinical trials are still necessary for generating reliable evidence with which to support these treatments. We suggest that the administration of just-sufficient doses of viable cells in appropriate regimens is critical to achieve effective and durable effects. In terms of future perspectives, genetic modification, complex products with extracellular vesicles derived from MSCs, cell encapsulation in hydrogels, and 3D bioprinted tissue engineering are promising approaches with which to improve MSC therapies for OA.
Keywords: cartilage regeneration; clinical trials; intra-articular injection; mesenchymal stem cells; osteoarthritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Arden N., Cooper C. In: Osteoarthritis Handbook. Arden N., Cooper C., editors. Taylor and Francis; Abingdon, UK: 2006.
-
- Jiang Y., Jahagirdar B.N., Reinhardt R.L., Schwartz R.E., Keene C.D., Ortiz-Gonzalez X.R., Reyes M., Lenvik T., Lund T., Blackstad M., et al. Erratum: Pluripotency of Mesenchymal Stem Cells Derived from Adult Marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. Erratum in Nature 2007, 447, 879–880. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

