Gold-Based Nanostructures for Antibacterial Application
- PMID: 37373154
- PMCID: PMC10297978
- DOI: 10.3390/ijms241210006
Gold-Based Nanostructures for Antibacterial Application
Abstract
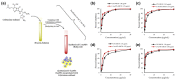
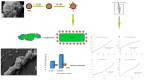
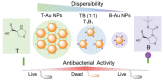
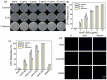
Bacterial infections have become a fatal threat because of the abuse of antibiotics in the world. Various gold (Au)-based nanostructures have been extensively explored as antibacterial agents to combat bacterial infections based on their remarkable chemical and physical characteristics. Many Au-based nanostructures have been designed and their antibacterial activities and mechanisms have been further examined and demonstrated. In this review, we collected and summarized current developments of antibacterial agents of Au-based nanostructures, including Au nanoparticles (AuNPs), Au nanoclusters (AuNCs), Au nanorods (AuNRs), Au nanobipyramids (AuNBPs), and Au nanostars (AuNSs) according to their shapes, sizes, and surface modifications. The rational designs and antibacterial mechanisms of these Au-based nanostructures are further discussed. With the developments of Au-based nanostructures as novel antibacterial agents, we also provide perspectives, challenges, and opportunities for future practical clinical applications.
Keywords: antibacterial mechanism; bacterial infection; gold nanobipyramids; gold nanoclusters; gold nanoparticles; gold nanorods; gold nanostars.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Zhu Y.P., Kuo T.R., Li Y.H., Qi M.Y., Chen G., Wang J.L., Xu Y.J., Chen H.M. Emerging dynamic structure of electrocatalysts unveiled by in situ X-ray diffraction/absorption spectroscopy. Energy Environ. Sci. 2021;14:1928–1958. doi: 10.1039/D0EE03903A. - DOI
-
- Okoro G., Husain S., Saukani M., Mutalik C., Yougbaré S., Hsiao Y.-C., Kuo T.-R. Emerging trends in nanomaterials for photosynthetic biohybrid systems. ACS Mater. Lett. 2023;5:95–115. doi: 10.1021/acsmaterialslett.2c00752. - DOI
-
- Kuo T.-R., Liao H.-J., Chen Y.-T., Wei C.-Y., Chang C.-C., Chen Y.-C., Chang Y.-H., Lin J.-C., Lee Y.-C., Wen C.-Y. Extended visible to near-infrared harvesting of earth-abundant FeS2-TiO2 heterostructures for highly active photocatalytic hydrogen evolution. Green Chem. 2018;20:1640–1647. doi: 10.1039/C7GC03173D. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

