Decipher the Immunopathological Mechanisms and Set Up Potential Therapeutic Strategies for Patients with Lupus Nephritis
- PMID: 37373215
- PMCID: PMC10298725
- DOI: 10.3390/ijms241210066
Decipher the Immunopathological Mechanisms and Set Up Potential Therapeutic Strategies for Patients with Lupus Nephritis
Abstract
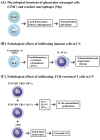
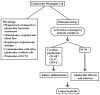
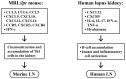
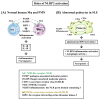
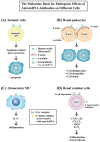
Lupus nephritis (LN) is one of the most severe complications in patients with systemic lupus erythematosus (SLE). Traditionally, LN is regarded as an immune complex (IC) deposition disease led by dsDNA-anti-dsDNA-complement interactions in the subendothelial and/or subepithelial basement membrane of glomeruli to cause inflammation. The activated complements in the IC act as chemoattractants to chemically attract both innate and adaptive immune cells to the kidney tissues, causing inflammatory reactions. However, recent investigations have unveiled that not only the infiltrating immune-related cells, but resident kidney cells, including glomerular mesangial cells, podocytes, macrophage-like cells, tubular epithelial cells and endothelial cells, may also actively participate in the inflammatory and immunological reactions in the kidney. Furthermore, the adaptive immune cells that are infiltrated are genetically restricted to autoimmune predilection. The autoantibodies commonly found in SLE, including anti-dsDNA, are cross-reacting with not only a broad spectrum of chromatin substances, but also extracellular matrix components, including α-actinin, annexin II, laminin, collagen III and IV, and heparan sulfate proteoglycan. Besides, the glycosylation on the Fab portion of IgG anti-dsDNA antibodies can also affect the pathogenic properties of the autoantibodies in that α-2,6-sialylation alleviates, whereas fucosylation aggravates their nephritogenic activity. Some of the coexisting autoantibodies, including anti-cardiolipin, anti-C1q, anti-ribosomal P autoantibodies, may also enhance the pathogenic role of anti-dsDNA antibodies. In clinical practice, the identification of useful biomarkers for diagnosing, monitoring, and following up on LN is quite important for its treatments. The development of a more specific therapeutic strategy to target the pathogenic factors of LN is also critical. We will discuss these issues in detail in the present article.
Keywords: NLRP3 inflammasome; anti-dsDNA antibodies; cross-reactivity; lupus nephritis; renal resident cells; type I interferon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hanly J.G., O’Keeffe A.G., Su L., Urowitz M.B., Romero-Diaz J., Gordon C., Bae S.-C., Bernatsky S., Clarke A.E., Wallace D.J., et al. The frequency and outcome of lupus nephritis: Results from an international inception cohort study. Rheumatology. 2016;55:252–262. doi: 10.1093/rheumatology/kev311. - DOI - PMC - PubMed
-
- Weening J.J., D’Agati V.D., Schwartz M.M., Seshan S.V., Alpers C.E., Appel G.B., Balow J.E., Bruijn J.A., Cook T., Ferrario F., et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65:521–530. doi: 10.1111/j.1523-1755.2004.00443.x. - DOI - PubMed
-
- Bastian H.M., Roseman J.M., McGwin G., Jr., Alarcón G.S., Friedman A.W., Fessler B.J., Baethge B.A., Reveille J.D., LUMINA Study Group Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus. 2002;11:152–160. doi: 10.1191/0961203302lu158oa. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

