Endothelial Cells and Mitochondria: Two Key Players in Liver Transplantation
- PMID: 37373238
- PMCID: PMC10298511
- DOI: 10.3390/ijms241210091
Endothelial Cells and Mitochondria: Two Key Players in Liver Transplantation
Abstract
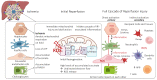
Building the inner layer of our blood vessels, the endothelium forms an important line communicating with deeper parenchymal cells in our organs. Previously considered passive, endothelial cells are increasingly recognized as key players in intercellular crosstalk, vascular homeostasis, and blood fluidity. Comparable to other cells, their metabolic function strongly depends on mitochondrial health, and the response to flow changes observed in endothelial cells is linked to their mitochondrial metabolism. Despite the direct impact of new dynamic preservation concepts in organ transplantation, the impact of different perfusion conditions on sinusoidal endothelial cells is not yet explored well enough. This article therefore describes the key role of liver sinusoidal endothelial cells (LSECs) together with their mitochondrial function in the context of liver transplantation. The currently available ex situ machine perfusion strategies are described with their effect on LSEC health. Specific perfusion conditions, including perfusion pressure, duration, and perfusate oxygenation are critically discussed considering the metabolic function and integrity of liver endothelial cells and their mitochondria.
Keywords: endothelial cells; ischemia-reperfusion injury; machine perfusion; mitochondria; reactive oxygen species; shears stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hong S.-G., Shin J., Choi S.Y., Powers J.C., Meister B.M., Sayoc J., Son J.S., Tierney R., Recchia F.A., Brown M.D., et al. Flow pattern–dependent mitochondrial dynamics regulates the metabolic profile and inflammatory state of endothelial cells. J. Clin. Investig. 2022;7:e159286. doi: 10.1172/jci.insight.159286. - DOI - PMC - PubMed
-
- Hofmann J., Otarashvili G., Meszaros A., Ebner S., Weissenbacher A., Cardini B., Oberhuber R., Resch T., Öfner D., Schneeberger S., et al. Restoring Mitochondrial Function While Avoiding Redox Stress: The Key to Preventing Ischemia/Reperfusion Injury in Machine Perfused Liver Grafts? Int. J. Mol. Sci. 2020;21:3132. doi: 10.3390/ijms21093132. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

