Effect of Purslane (Portulaca oleracea L.) on Intestinal Morphology, Digestion Activity and Microbiome of Chinese Pond Turtle (Mauremys reevesii) during Aeromonas hydrophila Infection
- PMID: 37373406
- PMCID: PMC10298896
- DOI: 10.3390/ijms241210260
Effect of Purslane (Portulaca oleracea L.) on Intestinal Morphology, Digestion Activity and Microbiome of Chinese Pond Turtle (Mauremys reevesii) during Aeromonas hydrophila Infection
Abstract
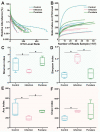
Large-scale mortality due to Aeromonas hydrophila (A. hydrophila) infection has considerably decreased the yield of the Chinese pond turtle (Mauremys reevesii). Purslane is a naturally active substance with a wide range of pharmacological functions, but its antibacterial effect on Chinese pond turtles infected by A. hydrophila infection is still unknown. In this study, we investigated the effect of purslane on intestinal morphology, digestion activity, and microbiome of Chinese pond turtles during A. hydrophila infection. The results showed that purslane promoted epidermal neogenesis of the limbs and increased the survival and feeding rates of Chinese pond turtles during A. hydrophila infection. Histopathological observation and enzyme activity assay indicated that purslane improved the intestinal morphology and digestive enzyme (α-amylase, lipase and pepsin) activities of Chinese pond turtle during A. hydrophila infection. Microbiome analysis revealed that purslane increased the diversity of intestinal microbiota with a significant decrease in the proportion of potentially pathogenic bacteria (such as Citrobacter freundii, Eimeria praecox, and Salmonella enterica) and an increase in the abundance of probiotics (such as uncultured Lactobacillus). In conclusion, our study uncovers that purslane improves intestinal health to protect Chinese pond turtles against A. hydrophila infection.
Keywords: A. hydrophila; Chinese pond turtle; intestine; microbiome; purslane.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Tekedar H.C., Waldbieser G.C., Karsi A., Liles M.R., Griffin M.J., Vamenta S., Sonstegard T., Hossain M., Schroeder S.G., Khoo L., et al. Complete Genome Sequence of a Channel Catfish Epidemic Isolate, A. hydrophila Strain ML09-119. Genome Announc. 2013;1:e00755-13. doi: 10.1128/genomeA.00755-13. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

