Potential Antitumor Effect of α-Mangostin against Rat Mammary Gland Tumors Induced by LA7 Cells
- PMID: 37373429
- PMCID: PMC10299034
- DOI: 10.3390/ijms241210283
Potential Antitumor Effect of α-Mangostin against Rat Mammary Gland Tumors Induced by LA7 Cells
Abstract
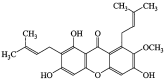
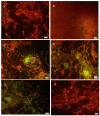
In this study, the chemotherapeutic effect of α-mangostin (AM) was assessed in rats injected with LA7 cells. Rats received AM orally at 30 and 60 mg/kg twice a week for 4 weeks. Cancer biomarkers such as CEA and CA 15-3 were significantly lower in AM-treated rats. Histopathological evaluations showed that AM protects the rat mammary gland from the carcinogenic effects of LA7 cells. Interestingly, AM decreased lipid peroxidation and increased antioxidant enzymes when compared to the control. Immunohistochemistry results of the untreated rats showed abundant PCNA and fewer p53-positive cells than AM-treated rats. Using the TUNEL test, AM-treated animals had higher apoptotic cell numbers than those untreated. This report revealed that that AM lessened oxidative stress, suppressed proliferation, and minimized LA7-induced mammary carcinogenesis. Therefore, the current study suggests that AM has significant potential for breast cancer treatment.
Keywords: LA7 cells; antioxidant; apoptosis; immunohistochemistry; mammary cancer; α-mangostin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Feng Y., Spezia M., Huang S., Yuan C., Zeng Z., Zhang L., Ji X., Liu W., Huang B., Luo W., et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5:77–106. doi: 10.1016/j.gendis.2018.05.001. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

