Impact of Manganese and Chromate on Specific DNA Double-Strand Break Repair Pathways
- PMID: 37373538
- PMCID: PMC10298927
- DOI: 10.3390/ijms241210392
Impact of Manganese and Chromate on Specific DNA Double-Strand Break Repair Pathways
Abstract
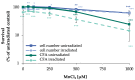
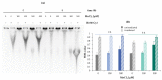
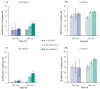
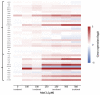
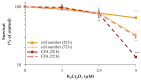
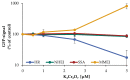
Manganese is an essential trace element; nevertheless, on conditions of overload, it becomes toxic, with neurotoxicity being the main concern. Chromate is a well-known human carcinogen. The underlying mechanisms seem to be oxidative stress as well as direct DNA damage in the case of chromate, but also interactions with DNA repair systems in both cases. However, the impact of manganese and chromate on DNA double-strand break (DSB) repair pathways is largely unknown. In the present study, we examined the induction of DSB as well as the effect on specific DNA DSB repair mechanisms, namely homologous recombination (HR), non-homologous end joining (NHEJ), single strand annealing (SSA), and microhomology-mediated end joining (MMEJ). We applied DSB repair pathway-specific reporter cell lines, pulsed field gel electrophoresis as well as gene expression analysis, and investigated the binding of specific DNA repair proteins via immunoflourescence. While manganese did not seem to induce DNA DSB and had no impact on NHEJ and MMEJ, HR and SSA were inhibited. In the case of chromate, the induction of DSB was further supported. Regarding DSB repair, no inhibition was seen in the case of NHEJ and SSA, but HR was diminished and MMEJ was activated in a pronounced manner. The results indicate a specific inhibition of error-free HR by manganese and chromate, with a shift towards error-prone DSB repair mechanisms in both cases. These observations suggest the induction of genomic instability and may explain the microsatellite instability involved in chromate-induced carcinogenicity.
Keywords: BRCA1; DNA DSB repair; HR; MMEJ; NHEJ; RAD51; RAD54; SSA; U2OS reporter-assay; chromate; manganese.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- IARC . IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Volume 49. World Health Organization; Lyon, France: 1990. Chromium and chromium compounds; pp. 49–256.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

