Association of Sodium-Glucose Cotransporter 2 Inhibitors with Osteomyelitis and Other Lower Limb Safety Outcomes in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomised Controlled Trials
- PMID: 37373652
- PMCID: PMC10299360
- DOI: 10.3390/jcm12123958
Association of Sodium-Glucose Cotransporter 2 Inhibitors with Osteomyelitis and Other Lower Limb Safety Outcomes in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomised Controlled Trials
Abstract
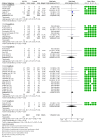
Our aim was to evaluate osteomyelitis and other major lower limb safety outcomes (i.e., peripheral artery disease or PAD, ulcers, atraumatic fractures, amputations, symmetric polyneuropathy, and infections) in patients affected by type 2 diabetes mellitus (T2DM) and treated with sodium-glucose cotransporter 2 inhibitors (SGLT2-is). We thus performed a systematic review and meta-analysis of randomised controlled trials (RCTs) comparing SGLT2-is at approved doses for T2DM with a placebo or standard of care. MEDLINE, Embase, and Cochrane CENTRAL were searched through August 2022. Separate intention-to-treat analyses were implemented for each molecule to calculate Mantel-Haenszel risk ratios (RRMH) with 95% confidence intervals (CIs) through a random-effects model. We processed data from 42 RCTs for a total of 29,491 and 23,052 patients, respectively assigned to SGLT2-i and comparator groups. SGLT2-is showed a pooled neutral effect on osteomyelitis, PAD, fractures, and symmetric polyneuropathy, whereas slightly deleterious sway on ulcers (RRMH 1.39 [1.01-1.91]), amputations (RRMH 1.27 [1.04-1.55]), and infections (RRMH 1.20 [1.02-1.40]). In conclusion, SGLT2-is appear to not significantly interfere with the onset of osteomyelitis, PAD, lower limb fractures, or symmetric polyneuropathy, even though the number of these events proved consistently higher in the investigational groups; otherwise, local ulcers, amputations, and overall infections may be favoured by their employment. This study is registered with the Open Science Framework (OSF).
Keywords: SGLT2 inhibitors; drug safety; lower limb complications; meta-analysis; osteomyelitis; type 2 diabetes mellitus.
Conflict of interest statement
B.P. received both honoraria as a speaker from Novo Nordisk and Eli Lilly and funds for serving on the MSD advisory board. F.B. reports funds for serving on the BD advisory board and received honoraria as a speaker from Eli Lilly. The remaining authors declare no competing interests.
Figures








References
-
- Salah H.M., Al’aref S.J., Khan M.S., Al-Hawwas M., Vallurupalli S., Mehta J.L., Mounsey J.P., Greene S.J., McGuire D.K., Lopes R.D., et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors initiation in patients with acute heart failure, with and without type 2 diabetes: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2022;21:20. doi: 10.1186/s12933-022-01455-2. - DOI - PMC - PubMed
-
- Donnan J.R., Grandy C.A., Chibrikov E., Marra C.A., Aubrey-Bassler K., Johnston K., Swab M., Hache J., Curnew D., Nguyen H., et al. Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: A systematic review and meta-analysis. BMJ Open. 2019;9:e022577. doi: 10.1136/bmjopen-2018-022577. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

