MGMT Promoter Methylation: Prognostication beyond Treatment Response
- PMID: 37373988
- PMCID: PMC10302387
- DOI: 10.3390/jpm13060999
MGMT Promoter Methylation: Prognostication beyond Treatment Response
Abstract
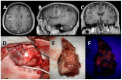
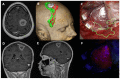
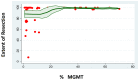
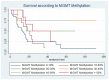
MGMT promoter methylation is related to the increased sensitivity of tumour tissue to chemotherapy with temozolomide (TMZ) and thus to improved patient survival. However, it is unclear how the extent of MGMT promoter methylation affects outcomes. In our study, a single-centre retrospective study, we explore the impact of MGMT promoter methylation in patients with glioblastoma who were operated upon with 5-ALA. Demographic, clinical and histology data, and survival rates were assessed. A total of 69 patients formed the study group (mean age 53.75 ± 15.51 years old). Positive 5-ALA fluorescence was noted in 79.41%. A higher percentage of MGMT promoter methylation was related to lower preoperative tumour volume (p = 0.003), a lower likelihood of 5-ALA positive fluorescence (p = 0.041) and a larger extent of resection EoR (p = 0.041). A higher MGMT promoter methylation rate was also related to improved progression-free survival (PFS) and overall survival (OS) (p = 0.008 and p = 0.006, respectively), even when adjusted for the extent of resection (p = 0.034 and p = 0.042, respectively). A higher number of adjuvant chemotherapy cycles was also related to longer PFS and OS (p = 0.049 and p = 0.030, respectively). Therefore, this study suggests MGMT promoter methylation should be considered as a continuous variable. It is a prognostic factor that goes beyond sensitivity to chemotherapy treatment, as a higher percentage of methylation is related not only to increased EoR and increased PFS and OS, but also to lower tumour volume at presentation and a lower likelihood of 5-ALA fluorescence intraoperatively.
Keywords: 5-ALA; GBM; MGMT; glioblastoma; methylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
-
- Ostrom Q.T., Gittleman H., Farah P., Ondracek A., Chen Y., Wolinsky Y., Stroup N.E., Kruchko C., Barnholtz-Sloan J.S. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2006–2010. Neuro-Oncology. 2013;15((Suppl. 2)):ii1–ii56. doi: 10.1093/neuonc/not151. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

