Spinal Cord Organoids to Study Motor Neuron Development and Disease
- PMID: 37374039
- PMCID: PMC10303776
- DOI: 10.3390/life13061254
Spinal Cord Organoids to Study Motor Neuron Development and Disease
Abstract
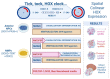
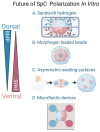
Motor neuron diseases (MNDs) are a heterogeneous group of disorders that affect the cranial and/or spinal motor neurons (spMNs), spinal sensory neurons and the muscular system. Although they have been investigated for decades, we still lack a comprehensive understanding of the underlying molecular mechanisms; and therefore, efficacious therapies are scarce. Model organisms and relatively simple two-dimensional cell culture systems have been instrumental in our current knowledge of neuromuscular disease pathology; however, in the recent years, human 3D in vitro models have transformed the disease-modeling landscape. While cerebral organoids have been pursued the most, interest in spinal cord organoids (SCOs) is now also increasing. Pluripotent stem cell (PSC)-based protocols to generate SpC-like structures, sometimes including the adjacent mesoderm and derived skeletal muscle, are constantly being refined and applied to study early human neuromuscular development and disease. In this review, we outline the evolution of human PSC-derived models for generating spMN and recapitulating SpC development. We also discuss how these models have been applied to exploring the basis of human neurodevelopmental and neurodegenerative diseases. Finally, we provide an overview of the main challenges to overcome in order to generate more physiologically relevant human SpC models and propose some exciting new perspectives.
Keywords: development; in vitro disease modeling; induced pluripotent stem cells (iPSCs); motor neuron (MN); motor neuron diseases (MNDs); organoids; spinal cord (SpC); spinal cord organoids (SCOs).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kadoshima T., Sakaguchi H., Nakano T., Soen M., Ando S., Eiraku M., Sasai Y. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell–derived neocortex. Proc. Natl. Acad. Sci. USA. 2013;110:20284–20289. doi: 10.1073/pnas.1315710110. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

