Serpentinization-Associated Mineral Catalysis of the Protometabolic Formose System
- PMID: 37374080
- PMCID: PMC10303151
- DOI: 10.3390/life13061297
Serpentinization-Associated Mineral Catalysis of the Protometabolic Formose System
Abstract
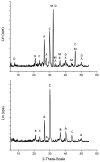
The formose reaction is a plausible prebiotic chemistry, famed for its production of sugars. In this work, we demonstrate that the Cannizzaro process is the dominant process in the formose reaction under many different conditions, thus necessitating a catalyst for the formose reaction under various environmental circumstances. The investigated formose reactions produce primarily organic acids associated with metabolism, a protometabolic system, and yield very little sugar left over. This is due to many of the acids forming from the degradation and Cannizaro reactions of many of the sugars produced during the formose reaction. We also show the heterogeneous Lewis-acid-based catalysis of the formose reaction by mineral systems associated with serpentinization. The minerals that showed catalytic activity include olivine, serpentinite, and calcium, and magnesium minerals including dolomite, calcite, and our Ca/Mg-chemical gardens. In addition, computational studies were performed for the first step of the formose reaction to investigate the reaction of formaldehyde, to either form methanol and formic acid under a Cannizzaro reaction or to react to form glycolaldehyde. Here, we postulate that serpentinization is therefore the startup process necessary to kick off a simple proto metabolic system-the formose protometabolic system.
Keywords: chemical complexity; chemical evolution; chemical garden; formose reaction; hydrothermal environments; metabolism; prebiotic chemistry; serpentinization; systems chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
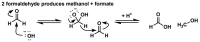




References
-
- Yadav M., Yadav H.S., editors. Biochemistry: Fundamentals and Bioenergetics. Bentham Science Publishers; Sharjah, United Arab Emirates: 2021.
-
- Breslow R. On the mechanism of the formose reaction. Tetrahedron Lett. 1959;1:22–26. doi: 10.1016/S0040-4039(01)99487-0. - DOI
LinkOut - more resources
Full Text Sources

