Pd-Ceria/CNMs Composites as Catalysts for CO and CH4 Oxidation
- PMID: 37374441
- PMCID: PMC10302758
- DOI: 10.3390/ma16124257
Pd-Ceria/CNMs Composites as Catalysts for CO and CH4 Oxidation
Abstract
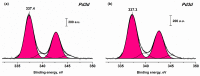
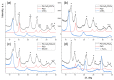
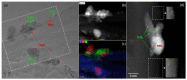
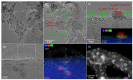
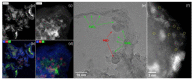
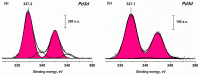
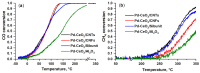
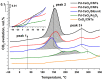
The application of composite materials as catalysts for the oxidation of CO and other toxic compounds is a promising approach for air purification. In this work, the composites comprising palladium and ceria components supported on multiwall carbon nanotubes, carbon nanofibers and Sibunit were studied in the reactions of CO and CH4 oxidation. The instrumental methods showed that the defective sites of carbon nanomaterials (CNMs) successfully stabilize the deposited components in a highly-dispersed state: PdO and CeO2 nanoparticles, subnanosized PdOx and PdxCe1-xO2-δ clusters with an amorphous structure, as well as single Pd and Ce atoms, are formed. It was shown that the reactant activation process occurs on palladium species with the participation of oxygen from the ceria lattice. The presence of interblock contacts between PdO and CeO2 nanoparticles has an important effect on oxygen transfer, which consequently affects the catalytic activity. The morphological features of the CNMs, as well as the defect structure, have a strong influence on the particle size and mutual stabilization of the deposited PdO and CeO2 components. The optimal combination of highly dispersed PdOx and PdxCe1-xO2-δ species, as well as PdO nanoparticles in the CNTs-based catalyst, makes it highly effective in both studied oxidation reactions.
Keywords: CO oxidation; carbon nanomaterials; ceria; composite catalysts; palladium.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- Yao Y.-F.Y. The Oxidation of CO and Hydrocarbons over Noble Metal Catalysts. J. Catal. 1984;87:152–162. doi: 10.1016/0021-9517(84)90178-7. - DOI
-
- Farrauto R.J., Deeba M., Alerasool S. Gasoline Automobile Catalysis and Its Historical Journey to Cleaner Air. Nat. Catal. 2019;2:603–613. doi: 10.1038/s41929-019-0312-9. - DOI
-
- Al Soubaihi R., Saoud K., Dutta J. Critical Review of Low-Temperature CO Oxidation and Hysteresis Phenomenon on Heterogeneous Catalysts. Catalysts. 2018;8:660. doi: 10.3390/catal8120660. - DOI
-
- Kim H.J., Shin D., Jeong H., Jang M.G., Lee H., Han J.W. Design of an Ultrastable and Highly Active Ceria Catalyst for CO Oxidation by Rare-Earth- and Transition-Metal Co-Doping. ACS Catal. 2020;10:14877–14886. doi: 10.1021/acscatal.0c03386. - DOI
-
- Masui T., Ozaki T., Machida K., Adachi G. Preparation of Ceria–Zirconia Sub-Catalysts for Automotive Exhaust Cleaning. J. Alloys Compd. 2000;303–304:49–55. doi: 10.1016/S0925-8388(00)00603-4. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

