Freeze-Drying Process for the Fabrication of Collagen-Based Sponges as Medical Devices in Biomedical Engineering
- PMID: 37374608
- PMCID: PMC10302432
- DOI: 10.3390/ma16124425
Freeze-Drying Process for the Fabrication of Collagen-Based Sponges as Medical Devices in Biomedical Engineering
Abstract
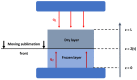
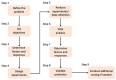
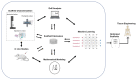
This paper presents a systematic review of a key sector of the much promising and rapidly evolving field of biomedical engineering, specifically on the fabrication of three-dimensional open, porous collagen-based medical devices, using the prominent freeze-drying process. Collagen and its derivatives are the most popular biopolymers in this field, as they constitute the main components of the extracellular matrix, and therefore exhibit desirable properties, such as biocompatibility and biodegradability, for in vivo applications. For this reason, freeze-dried collagen-based sponges with a wide variety of attributes can be produced and have already led to a wide range of successful commercial medical devices, chiefly for dental, orthopedic, hemostatic, and neuronal applications. However, collagen sponges display some vulnerabilities in other key properties, such as low mechanical strength and poor control of their internal architecture, and therefore many studies focus on the settlement of these defects, either by tampering with the steps of the freeze-drying process or by combining collagen with other additives. Furthermore, freeze drying is still considered a high-cost and time-consuming process that is often used in a non-optimized manner. By applying an interdisciplinary approach and combining advances in other technological fields, such as in statistical analysis, implementing the Design of Experiments, and Artificial Intelligence, the opportunity arises to further evolve this process in a sustainable and strategic manner, and optimize the resulting products as well as create new opportunities in this field.
Keywords: Artificial Intelligence; alignment; biomaterials; biomedical engineering; collagen; freeze drying; gelatin; medical devices; modeling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Zhang X.-Y. Biomedical engineering for health research and development. Eur. Rev. Med. Pharm. Sci. 2015;19:220–224. - PubMed
-
- Christy P.N., Basha S.K., Kumari V.S., Bashir A.K.H., Maaza M., Kaviyarasu K., Arasu M.V., Al-Dhabi N.A., Ignacimuthu S. Biopolymeric nanocomposite scaffolds for bone tissue engineering applications—A review. J. Drug Deliv. Sci. Technol. 2020;55:101452. doi: 10.1016/j.jddst.2019.101452. - DOI
-
- Abdelaziz A.G., Nageh H., Abdo S.M., Abdalla M.S., Amer A.A., Abdal-hay A., Barhoum A. A Review of 3D Polymeric Scaffolds for Bone Tissue Engineering: Principles, Fabrication Techniques, Immunomodulatory Roles, and Challenges. Bioengineering. 2023;10:204. doi: 10.3390/bioengineering10020204. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

