Epigenetic Induction of Secondary Metabolites Production in Endophytic Fungi Penicillium chrysogenum and GC-MS Analysis of Crude Metabolites with Anti-HIV-1 Activity
- PMID: 37374906
- PMCID: PMC10305084
- DOI: 10.3390/microorganisms11061404
Epigenetic Induction of Secondary Metabolites Production in Endophytic Fungi Penicillium chrysogenum and GC-MS Analysis of Crude Metabolites with Anti-HIV-1 Activity
Abstract
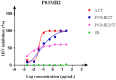
The continuous burden of human immunodeficiency virus-1 in Sub-Saharan Africa, coupled with the inability of antiretroviral agents to eradicate HIV-1 from viral reservoirs, the potential risks of drug resistance development, and the development of adverse effects, emphasizes the need to develop a new class of HIV-1 inhibitors. Here, we cultivated four endophytic fungal isolates from a medicinal plant, Albizia adianthifolia with the addition of small epigenetic modifiers, sodium butyrate, and valproic acid, to induce the expression of biosynthetic gene clusters encoding active secondary metabolites with probable anti-HIV activities. We identified a non-toxic crude extract of the endophytic fungus Penicillium chrysogenum treated with sodium butyrate to possess significantly greater anti-HIV activity than the untreated extracts. Penicillium chrysogenum P03MB2 showed anti-HIV activity with an IC50 of 0.6024 µg/mL compared to untreated fungal crude extract (IC50 5.053 µg/mL) when treated with sodium butyrate. The profile of secondary metabolite compounds from the bioactive, partially purified extracts were identified by gas chromatography-mass spectrometry (GC-MS), and more bioactive compounds were detected in treated P. chrysogenum P03MB2 fractions than in untreated fractions. Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro (13.64%), cyclotrisiloxane, hexamethyl (8.18%), cyclotetrasiloxane, octamethyl (7.23%), cyclopentasiloxane, decamethyl (6.36%), quinoline, 1,2-dihydro-2,24-trimethyl (5.45%), propanenitrile (4.55%), deca-6,9-diene (4.55%), dibutyl phthalate (4.55%), and silane[1,1-dimethyl-2-propenyl)oxy]dimethyl (2.73%) were the most abundant compounds. These results indicate that treatment of endophytic fungi with small epigenetic modifiers enhances the secretion of secondary metabolites with stronger anti-HIV-1 properties, acknowledging the feasibility of epigenetic modification as an innovative approach for the discovery of cryptic fungal metabolites which can be developed into therapeutic compounds.
Keywords: biosynthetic gene clusters; endophytic fungi; epigenetic modifiers; human immunodeficiency virus; secondary metabolites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

