Faecal Microbiota Characterisation of Potamochoerus porcus Living in a Controlled Environment
- PMID: 37375044
- PMCID: PMC10305278
- DOI: 10.3390/microorganisms11061542
Faecal Microbiota Characterisation of Potamochoerus porcus Living in a Controlled Environment
Abstract
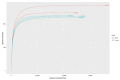
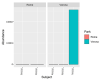
Intestinal bacteria establish a specific relationship with the host animal, which causes the acquisition of gut microbiota with a unique composition classified as the enterotype. As the name suggests, the Red River Hog is a wild member of the pig family living in Africa, in particular through the West and Central African rainforest. To date, very few studies have analysed the gut microbiota of Red River Hogs (RRHs) both housed under controlled conditions and in wild habitats. This study analysed the intestinal microbiota and the distribution of Bifidobacterium species in five Red River Hog (RRH) individuals (four adults and one juvenile), hosted in two different modern zoological gardens (Parco Natura Viva, Verona, and Bioparco, Rome) with the aim of disentangling the possible effects of captive different lifestyle and host genetics. Faecal samples were collected and studied both for bifidobacterial counts and isolation by means of culture-dependent method and for total microbiota analysis through the high-quality sequences of the V3-V4 region of bacterial 16S rRNA. Results showed a host-specific bifidobacterial species distribution. Indeed, B. boum and B. thermoacidophilum were found only in Verona RRHs, whereas B. porcinum species were isolated only in Rome RRHs. These bifidobacterial species are also typical of pigs. Bifidobacterial counts were about 106 CFU/g in faecal samples of all the individuals, with the only exception for the juvenile subject, showing 107 CFU/g. As in human beings, in RRHs a higher count of bifidobacteria was also found in the young subject compared with adults. Furthermore, the microbiota of RRHs showed qualitative differences. Indeed, Firmicutes was found to be the dominant phylum in Verona RRHs whereas Bacteroidetes was the most represented in Roma RRHs. At order level, Oscillospirales and Spirochaetales were the most represented in Verona RRHs compared with Rome RRHs, where Bacteroidales dominated over the other taxa. Finally, at the family level, RRHs from the two sites showed the presence of the same families, but with different levels of abundance. Our results highlight that the intestinal microbiota seems to reflect the lifestyle (i.e., the diet), whereas age and host genetics are the driving factors for the bifidobacterial population.
Keywords: Potamocherus porcus; beneficial microbes; bifidobacteria; diet; gut microbiota.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials

