Antagonistic Interactions of Lactic Acid Bacteria from Human Oral Microbiome against Streptococcus mutans and Candida albicans
- PMID: 37375107
- PMCID: PMC10304193
- DOI: 10.3390/microorganisms11061604
Antagonistic Interactions of Lactic Acid Bacteria from Human Oral Microbiome against Streptococcus mutans and Candida albicans
Abstract
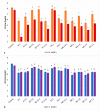
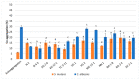
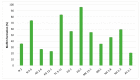
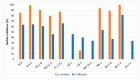
Oral probiotic lactic acid bacteria can exhibit antagonistic activities against pathogens associated with diseases in the oral cavity. Therefore, twelve previously isolated oral strains were assessed for antagonistic evaluation against selected oral test microorganisms Streptococcus mutans and Candida albicans. Two separate co-culturing analyses were performed, where all tested strains showed the presence of antagonistic activity and four strains, Limosilactobacillus fermentum N 2, TC 3-11, and NA 2-2, and Weissella confusa NN 1, significantly inhibited Streptococcus mutans by 3-5 logs. The strains showed antagonistic activity against Candida albicans, and all exhibited pathogen inhibition by up to 2 logs. Co-aggregation capability was assessed, showing co-aggregative properties with the selected pathogens. Biofilm formation and antibiofilm activity of the tested strains against the oral pathogens were assayed, where the strains showed specificity in self-biofilm formation and well-expressed antibiofilm properties by most of them above 79% and 50% against Streptococcus mutans and Candida albicans, respectively. The tested LAB strains were assayed by a KMnO4 antioxidant bioassay, where most of the native cell-free supernatants exhibited total antioxidant capacity. These results show that five tested strains are promising candidates to be included in new functional probiotic products for oral healthcare.
Keywords: Candida albicans; Streptococcus mutans; antagonistic activity; antioxidant activity; biofilm; lactic acid bacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Griessl T., Zechel-Gran S., Olejniczak S., Weigel M., Hain T., Domann E. High-resolution taxonomic examination of the oral microbiome after oil pulling with standardized sunflower seed oil and healthy participants: A pilot study. Clin. Oral Investig. 2021;25:2689–2703. doi: 10.1007/s00784-020-03582-0. - DOI - PMC - PubMed
-
- Santonocito S., Giudice A., Polizzi A., Troiano G., Merlo E.M., Sclafani R., Grosso G., Isola G. A Cross-Talk between Diet and the Oral Microbiome: Balance of Nutrition on Inflammation and Immune System’s Response during Periodontitis. Nutrients. 2022;14:2426. doi: 10.3390/nu14122426. - DOI - PMC - PubMed
-
- Alexa V.T., Galuscan A., Popescu I., Tirziu E., Obistioiu D., Floare A.D., Perdiou A., Jumanca D. Synergistic/Antagonistic Potential of Natural Preparations Based on Essential Oils Against Streptococcus mutans from the Oral Cavity. Molecules. 2019;24:4043. doi: 10.3390/molecules24224043. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

