Involvement of AoMdr1 in the Regulation of the Fluconazole Resistance, Mycelial Fusion, Conidiation, and Trap Formation of Arthrobotrys oligospora
- PMID: 37375114
- PMCID: PMC10302927
- DOI: 10.3390/microorganisms11061612
Involvement of AoMdr1 in the Regulation of the Fluconazole Resistance, Mycelial Fusion, Conidiation, and Trap Formation of Arthrobotrys oligospora
Abstract
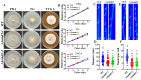
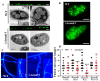
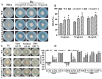
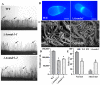
Multidrug resistance (Mdr) proteins are critical proteins for maintenance of drug resistance in fungi. Mdr1 has been extensively studied in Candida albicans; its role in other fungi is largely unknown. In this study, we identified a homologous protein of Mdr (AoMdr1) in the nematode-trapping (NT) fungus Arthrobotrys oligospora. It was found that the deletion of Aomdr1 resulted in a significant reduction in the number of hyphal septa and nuclei as well as increased sensitivity to fluconazole and resistance to hyperosmotic stress and SDS. The deletion of Aomdr1 also led to a remarkable increase in the numbers of traps and mycelial loops in the traps. Notably, AoMdr1 was able to regulate mycelial fusion under low-nutrient conditions, but not under nutrient-rich conditions. AoMdr1 was also involved in secondary metabolism, and its deletion caused an increase in arthrobotrisins (specific compounds produced by NT fungi). These results suggest that AoMdr1 plays a crucial role in the fluconazole resistance, mycelial fusion, conidiation, trap formation, and secondary metabolism of A. oligospora. Our study contributes to the understanding of the critical role of Mdr proteins in mycelial growth and the development of NT fungi.
Keywords: Arthrobotrys oligospora; conidiation; fluconazole resistance; multidrug resistance protein; trap formation.
Conflict of interest statement
We declare that we have no conflict of interest.
Figures

Similar articles
-
AoSte12 Is Required for Mycelial Development, Conidiation, Trap Morphogenesis, and Secondary Metabolism by Regulating Hyphal Fusion in Nematode-Trapping Fungus Arthrobotrys oligospora.Microbiol Spectr. 2023 Feb 14;11(2):e0395722. doi: 10.1128/spectrum.03957-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36786575 Free PMC article.
-
AoMedA has a complex regulatory relationship with AoBrlA, AoAbaA, and AoWetA in conidiation, trap formation, and secondary metabolism in the nematode-trapping fungus Arthrobotrys oligospora.Appl Environ Microbiol. 2023 Sep 28;89(9):e0098323. doi: 10.1128/aem.00983-23. Epub 2023 Sep 1. Appl Environ Microbiol. 2023. PMID: 37655869 Free PMC article.
-
AoMae1 Regulates Hyphal Fusion, Lipid Droplet Accumulation, Conidiation, and Trap Formation in Arthrobotrys oligospora.J Fungi (Basel). 2023 Apr 21;9(4):496. doi: 10.3390/jof9040496. J Fungi (Basel). 2023. PMID: 37108952 Free PMC article.
-
Recent Advances in Life History Transition with Nematode-Trapping Fungus Arthrobotrys oligospora and Its Application in Sustainable Agriculture.Pathogens. 2023 Feb 22;12(3):367. doi: 10.3390/pathogens12030367. Pathogens. 2023. PMID: 36986289 Free PMC article. Review.
-
Predator-prey interactions of nematode-trapping fungi and nematodes: both sides of the coin.Appl Microbiol Biotechnol. 2018 May;102(9):3939-3949. doi: 10.1007/s00253-018-8897-5. Epub 2018 Mar 9. Appl Microbiol Biotechnol. 2018. PMID: 29523933 Review.
Cited by
-
Multiple Roles of the Low-Affinity Calcium Uptake System in Drechslerella dactyloides, a Nematode-Trapping Fungus That Forms Constricting Rings.J Fungi (Basel). 2023 Sep 28;9(10):975. doi: 10.3390/jof9100975. J Fungi (Basel). 2023. PMID: 37888231 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

