Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment
- PMID: 37375116
- PMCID: PMC10305407
- DOI: 10.3390/microorganisms11061614
Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment
Erratum in
-
Correction: Sharma et al. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614.Microorganisms. 2024 Sep 27;12(10):1961. doi: 10.3390/microorganisms12101961. Microorganisms. 2024. PMID: 39458421 Free PMC article.
Abstract
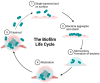
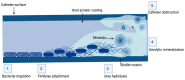
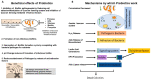
Biofilm is complex and consists of bacterial colonies that reside in an exopolysaccharide matrix that attaches to foreign surfaces in a living organism. Biofilm frequently leads to nosocomial, chronic infections in clinical settings. Since the bacteria in the biofilm have developed antibiotic resistance, using antibiotics alone to treat infections brought on by biofilm is ineffective. This review provides a succinct summary of the theories behind the composition of, formation of, and drug-resistant infections attributed to biofilm and cutting-edge curative approaches to counteract and treat biofilm. The high frequency of medical device-induced infections due to biofilm warrants the application of innovative technologies to manage the complexities presented by biofilm.
Keywords: antibiotic resistance; biofilm; biofilm control; extracellular polysaccharides; healthcare-associated infection; medical device infections.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

