Recent Configurational Advances for Solid-State Lithium Batteries Featuring Conversion-Type Cathodes
- PMID: 37375134
- PMCID: PMC10304597
- DOI: 10.3390/molecules28124579
Recent Configurational Advances for Solid-State Lithium Batteries Featuring Conversion-Type Cathodes
Abstract
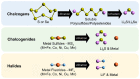
Solid-state lithium metal batteries offer superior energy density, longer lifespan, and enhanced safety compared to traditional liquid-electrolyte batteries. Their development has the potential to revolutionize battery technology, including the creation of electric vehicles with extended ranges and smaller more efficient portable devices. The employment of metallic lithium as the negative electrode allows the use of Li-free positive electrode materials, expanding the range of cathode choices and increasing the diversity of solid-state battery design options. In this review, we present recent developments in the configuration of solid-state lithium batteries with conversion-type cathodes, which cannot be paired with conventional graphite or advanced silicon anodes due to the lack of active lithium. Recent advancements in electrode and cell configuration have resulted in significant improvements in solid-state batteries with chalcogen, chalcogenide, and halide cathodes, including improved energy density, better rate capability, longer cycle life, and other notable benefits. To fully leverage the benefits of lithium metal anodes in solid-state batteries, high-capacity conversion-type cathodes are necessary. While challenges remain in optimizing the interface between solid-state electrolytes and conversion-type cathodes, this area of research presents significant opportunities for the development of improved battery systems and will require continued efforts to overcome these challenges.
Keywords: Li–S battery; all-solid-state battery; chalcogen cathode; chalcogenide cathode; fluoride cathode; halide cathode; metallic lithium anode; solid-state electrolyte; sulfide cathode; sulfur cathode.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Wu F., Yushin G. Conversion Cathodes for Rechargeable Lithium and Lithium-Ion Batteries. Energy Environ. Sci. 2017;10:435–459. doi: 10.1039/C6EE02326F. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

