Armchair Janus WSSe Nanotube Designed with Selenium Vacancy as a Promising Photocatalyst for CO2 Reduction
- PMID: 37375156
- PMCID: PMC10302939
- DOI: 10.3390/molecules28124602
Armchair Janus WSSe Nanotube Designed with Selenium Vacancy as a Promising Photocatalyst for CO2 Reduction
Abstract
Photocatalytic conversion of carbon dioxide into chemical fuels offers a promising way to not only settle growing environmental problems but also provide a renewable energy source. In this study, through first-principles calculation, we found that the Se vacancy introduction can lead to the transition of physical-to-chemical CO2 adsorption on Janus WSSe nanotube. Se vacancies work at the adsorption site, which significantly improves the amount of transferred electrons at the interface, resulting in the enhanced electron orbital hybridization between adsorbents and substrates, and promising the high activity and selectivity for carbon dioxide reduction reaction (CO2RR). Under the condition of illumination, due to the adequate driving forces of photoexcited holes and electrons, oxygen generation reaction (OER) and CO2RR can occur spontaneously on the S and Se sides of the defective WSSe nanotube, respectively. The CO2 could be reduced into CH4, meanwhile, the O2 is produced by the water oxidation, which also provides the hydrogen and electron source for the CO2RR. Our finding reveals a candidate photocatalyst for obtaining efficient photocatalytic CO2 conversion.
Keywords: CO2 reduction; Janus WSSe nanotube; Se vacancy; photocatalysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

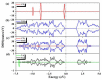

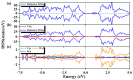




References
-
- Gattrell M., Gupta N., Co A. A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper. J. Electroanal. Chem. 2006;594:1–19. doi: 10.1016/j.jelechem.2006.05.013. - DOI
-
- Gattrell M., Gupta N., Co A. Electrochemical reduction of CO2 to hydrocarbons to store renewable electrical energy and upgrade biogas. Energy Convers. Manag. 2007;48:1255–1265. doi: 10.1016/j.enconman.2006.09.019. - DOI
-
- Wageh S., Al-Hartomy O.A., Alotaibi M.F., Liu L.-J. Ionized cocatalyst to promote CO2 photoreduction activity over core–triple-shell ZnO hollow spheres. Rare Met. 2022;41:1077–1079. doi: 10.1007/s12598-021-01902-1. - DOI
-
- Chen Y., Sun Q., Jena P. SiTe monolayers: Si-based analogues of phosphorene. J. Mater. Chem. C. 2016;4:6353–6361. doi: 10.1039/C6TC01138A. - DOI
-
- Modestra J.A., Mohan S.V. Microbial electrosynthesis of carboxylic acids through CO2 reduction with selectively enriched biocatalyst: Microbial dynamics. J. CO2 Util. 2017;20:190–199. doi: 10.1016/j.jcou.2017.05.011. - DOI
Grants and funding
- 232300420128/Natural Science Foundation of Henan Province
- 202210479032/National College Students Innovation and Entrepreneurship Training Program
- FSKFKT002/Open Project of Key Laboratory of Functional Materials and Devices for Informatics of Anhui Higher Education Institutes
- 202210479049/College Students Innovation Fund of Anyang Normal University
- 2022C01GX019/Key Scientific and Technological Projects in Anyang City
LinkOut - more resources
Full Text Sources

