An Accessible Method to Improve the Stability and Reusability of Porcine Pancreatic α-Amylase via Immobilization in Gellan-Based Hydrogel Particles Obtained by Ionic Cross-Linking with Mg2+ Ions
- PMID: 37375250
- PMCID: PMC10302431
- DOI: 10.3390/molecules28124695
An Accessible Method to Improve the Stability and Reusability of Porcine Pancreatic α-Amylase via Immobilization in Gellan-Based Hydrogel Particles Obtained by Ionic Cross-Linking with Mg2+ Ions
Abstract
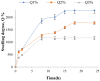
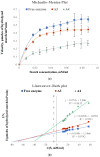
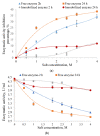
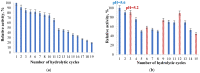
Amylase is an enzyme used to hydrolyze starch in order to obtain different products that are mainly used in the food industry. The results reported in this article refer to the immobilization of α-amylase in gellan hydrogel particles ionically cross-linked with Mg2+ ions. The obtained hydrogel particles were characterized physicochemically and morphologically. Their enzymatic activity was tested using starch as a substrate in several hydrolytic cycles. The results showed that the properties of the particles are influenced by the degree of cross-linking and the amount of immobilized α-amylase enzyme. The temperature and pH at which the immobilized enzyme activity is maximum were T = 60 °C and pH = 5.6. The enzymatic activity and affinity of the enzyme to the substrate depend on the particle type, and this decreases for particles with a higher cross-linking degree owing to the slow diffusion of the enzyme molecules inside the polymer's network. By immobilization, α-amylase is protected from environmental factors, and the obtained particles can be quickly recovered from the hydrolysis medium, thus being able to be reused in repeated hydrolytic cycles (at least 11 cycles) without a substantial decrease in enzymatic activity. Moreover, α-amylase immobilized in gellan particles can be reactivated via treatment with a more acidic medium.
Keywords: gellan hydrogel particles; hydrolysis; ionic cross-linking; magnesium acetate; α-amylase immobilized.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Preparation, characterization and stability studies of cross-linked α-amylase aggregates (CLAAs) for continuous liquefaction of starch.Int J Biol Macromol. 2021 Mar 15;173:267-276. doi: 10.1016/j.ijbiomac.2021.01.057. Epub 2021 Jan 14. Int J Biol Macromol. 2021. PMID: 33454331
-
Covalent immobilization of α-amylase on magnetic particles as catalyst for hydrolysis of high-amylose starch.Int J Biol Macromol. 2016 Jun;87:537-44. doi: 10.1016/j.ijbiomac.2016.02.080. Epub 2016 Mar 6. Int J Biol Macromol. 2016. PMID: 26959172
-
Efficient Immobilization of Porcine Pancreatic α-Amylase on Amino-Functionalized Magnetite Nanoparticles: Characterization and Stability Evaluation of the Immobilized Enzyme.Appl Biochem Biotechnol. 2016 Nov;180(5):954-968. doi: 10.1007/s12010-016-2145-1. Epub 2016 May 30. Appl Biochem Biotechnol. 2016. PMID: 27240662
-
Efficient hydrolysis of starch by α-amylase immobilized on cloisite 30B and modified forms of cloisite 30B by adsorption and covalent methods.Food Chem. 2022 Mar 30;373(Pt A):131425. doi: 10.1016/j.foodchem.2021.131425. Epub 2021 Oct 20. Food Chem. 2022. PMID: 34710686
-
Structure-based modification of a-amylase by conventional and emerging technologies: Comparative study on the secondary structure, activity, thermal stability and amylolysis efficiency.Food Chem. 2024 Mar 30;437(Pt 1):137903. doi: 10.1016/j.foodchem.2023.137903. Epub 2023 Oct 30. Food Chem. 2024. PMID: 37931423 Review.
Cited by
-
Degradable Polymeric Bio(nano)materials and Their Biomedical Applications: A Comprehensive Overview and Recent Updates.Polymers (Basel). 2024 Jan 10;16(2):206. doi: 10.3390/polym16020206. Polymers (Basel). 2024. PMID: 38257005 Free PMC article. Review.
-
Pharmaceutical Applications of Biomass Polymers: Review of Current Research and Perspectives.Polymers (Basel). 2024 Apr 23;16(9):1182. doi: 10.3390/polym16091182. Polymers (Basel). 2024. PMID: 38732651 Free PMC article. Review.
References
-
- Chapman J., Ismail A.E., Dinu C.Z. Industrial Applications of Enzymes: Recent Advances, Techniques, and Outlooks. Catalysts. 2018;8:238. doi: 10.3390/catal8060238. - DOI
-
- Karmee S.K. Moving towards the Application of Biocatalysis in Food Waste Biorefinery. Fermentation. 2023;9:73. doi: 10.3390/fermentation9010073. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

