The History of mARC
- PMID: 37375270
- PMCID: PMC10302757
- DOI: 10.3390/molecules28124713
The History of mARC
Abstract
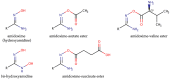
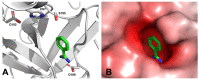
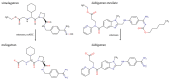
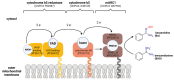
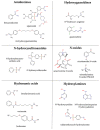
The mitochondrial amidoxime-reducing component (mARC) is the most recently discovered molybdoenzyme in humans after sulfite oxidase, xanthine oxidase and aldehyde oxidase. Here, the timeline of mARC's discovery is briefly described. The story begins with investigations into N-oxidation of pharmaceutical drugs and model compounds. Many compounds are N-oxidized extensively in vitro, but it turned out that a previously unknown enzyme catalyzes the retroreduction of the N-oxygenated products in vivo. After many years, the molybdoenzyme mARC could finally be isolated and identified in 2006. mARC is an important drug-metabolizing enzyme and N-reduction by mARC has been exploited very successfully for prodrug strategies, that allow oral administration of otherwise poorly bioavailable therapeutic drugs. Recently, it was demonstrated that mARC is a key factor in lipid metabolism and likely involved in the pathogenesis of non-alcoholic fatty liver disease (NAFLD). The exact link between mARC and lipid metabolism is not yet fully understood. Regardless, many now consider mARC a potential drug target for the prevention or treatment of liver diseases. This article focusses on discoveries related to mammalian mARC enzymes. mARC homologues have been studied in algae, plants and bacteria. These will not be discussed extensively here.
Keywords: biotransformation; mARC; molybdenum enzyme; prodrug; reductase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

