Protein Interactions in Rhodopseudomonas palustris TIE-1 Reveal the Molecular Basis for Resilient Photoferrotrophic Iron Oxidation
- PMID: 37375288
- PMCID: PMC10304953
- DOI: 10.3390/molecules28124733
Protein Interactions in Rhodopseudomonas palustris TIE-1 Reveal the Molecular Basis for Resilient Photoferrotrophic Iron Oxidation
Abstract
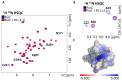
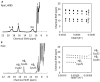
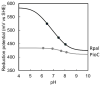
Rhodopseudomonas palustris is an alphaproteobacterium with impressive metabolic versatility, capable of oxidizing ferrous iron to fix carbon dioxide using light energy. Photoferrotrophic iron oxidation is one of the most ancient metabolisms, sustained by the pio operon coding for three proteins: PioB and PioA, which form an outer-membrane porin-cytochrome complex that oxidizes iron outside of the cell and transfers the electrons to the periplasmic high potential iron-sulfur protein (HIPIP) PioC, which delivers them to the light-harvesting reaction center (LH-RC). Previous studies have shown that PioA deletion is the most detrimental for iron oxidation, while, the deletion of PioC resulted in only a partial loss. The expression of another periplasmic HiPIP, designated Rpal_4085, is strongly upregulated in photoferrotrophic conditions, making it a strong candidate for a PioC substitute. However, it is unable to reduce the LH-RC. In this work we used NMR spectroscopy to map the interactions between PioC, PioA, and the LH-RC, identifying the key amino acid residues involved. We also observed that PioA directly reduces the LH-RC, and this is the most likely substitute upon PioC deletion. By contrast, Rpal_4085 demontrated significant electronic and structural differences from PioC. These differences likely explain its inability to reduce the LH-RC and highlight its distinct functional role. Overall, this work reveals the functional resilience of the pio operon pathway and further highlights the use of paramagnetic NMR for understanding key biological processes.
Keywords: HIPIP; Rhodopseudomonas; biological electron transfer; cytochrome c; paramagnetic NMR; photoferrotrophism; protein interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Konhauser K.O., Newman D.K., Kappler A. The Potential Significance of Microbial Fe(III) Reduction during Deposition of Precambrian Banded Iron Formations. Geobiology. 2005;3:167–177. doi: 10.1111/j.1472-4669.2005.00055.x. - DOI
-
- Guzman M.S., Rengasamy K., Binkley M.M., Jones C., Ranaivoarisoa T.O., Singh R., Fike D.A., Meacham J.M., Bose A. Phototrophic Extracellular Electron Uptake Is Linked to Carbon Dioxide Fixation in the Bacterium Rhodopseudomonas palustris. Nat. Commun. 2019;10:1355. doi: 10.1038/s41467-019-09377-6. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

