Stereochemistry of N-Acyl-5 H-dibenzo[ b, d]azepin-7(6 H)-ones
- PMID: 37375290
- PMCID: PMC10303827
- DOI: 10.3390/molecules28124734
Stereochemistry of N-Acyl-5 H-dibenzo[ b, d]azepin-7(6 H)-ones
Abstract
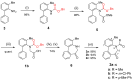
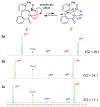
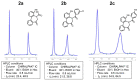
The stereochemical properties of N-acyl-5H-dibenzo[b,d]azepin-7(6H)-ones (2a-c), which inhibit potassium channels in T cells, were examined by freezing their conformational change due to 4-methyl substitution. N-Acyl-5H-dibenzo[b,d]azepin-7(6H)-ones exist as pairs of enantiomers [(a1R, a2R), (a1S, a2S)], and each atropisomer is separable at room temperature. An alternate procedure for preparing 5H-dibenzo[b,d]azepin-7(6H)-ones involves the intramolecular Friedel-Crafts cyclization of N-benzyloxycarbonylated biaryl amino acids. Consequently, the N-benzyloxy group was removed during the cyclization reaction to produce 5H-dibenzo[b,d]azepin-7(6H)-ones suitable for the subsequent N-acylation reaction.
Keywords: Kv1.3; atropisomer; axial chirality; dibenzoazepinone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Sheng F.-T., Yang S., Wu S.-F., Zhang Y.-C., Shi F. Catalytic asymmetric synthesis of axially chiral 3,3’-bisindoles by direct coupling of indole rings. Chin. J. Chem. 2022;40:2151–2160. doi: 10.1002/cjoc.202200327. - DOI
-
- Hang Q.-Q., Wu S.-F., Yang S., Wang X., Zhong Z., Zhang Y.-C. Design and catalytic atroposelective synthesis of axially chiral isochromenone-indoles. Sci. China Chem. 2022;65:1929–1937. doi: 10.1007/s11426-022-1363-y. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

