Morin Hydrate Encapsulation and Release from Mesoporous Silica Nanoparticles for Melanoma Therapy
- PMID: 37375331
- PMCID: PMC10302569
- DOI: 10.3390/molecules28124776
Morin Hydrate Encapsulation and Release from Mesoporous Silica Nanoparticles for Melanoma Therapy
Abstract
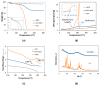
Melanoma incidence, a type of skin cancer, has been increasing worldwide. There is a strong need to develop new therapeutic strategies to improve melanoma treatment. Morin is a bioflavonoid with the potential for use in the treatment of cancer, including melanoma. However, therapeutic applications of morin are restrained owing to its low aqueous solubility and limited bioavailability. This work investigates morin hydrate (MH) encapsulation in mesoporous silica nanoparticles (MSNs) to enhance morin bioavailability and consequently increase the antitumor effects in melanoma cells. Spheroidal MSNs with a mean size of 56.3 ± 6.5 nm and a specific surface area of 816 m2/g were synthesized. MH was successfully loaded (MH-MSN) using the evaporation method, with a loading capacity of 28.3% and loading efficiency of 99.1%. In vitro release studies showed that morin release from MH-MSNs was enhanced at pH 5.2, indicating increased flavonoid solubility. The in vitro cytotoxicity of MH and MH-MSNs on human A375, MNT-1 and SK-MEL-28 melanoma cell lines was investigated. Exposure to MSNs did not affect the cell viability of any of the cell lines tested, suggesting that the nanoparticles are biocompatible. The effect of MH and MH-MSNs on reducing cell viability was time- and concentration-dependent in all melanoma cell lines. The A375 and SK-MEL-28 cell lines were slightly more sensitive than MNT-1 cells in both the MH and MH-MSN treatments. Our findings suggest that MH-MSNs are a promising delivery system for the treatment of melanoma.
Keywords: melanoma; mesoporous silica nanoparticles; morin; nano delivery system; nanotechnology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Garbe C., Amaral T., Peris K., Hauschild A., Arenberger P., Bastholt L., Bataille V., del Marmol V., Dréno B., Fargnoli M.C., et al. European Consensus-Based Interdisciplinary Guideline for Melanoma. Part 1: Diagnostics—Update 2019. Eur. J. Cancer. 2020;126:141–158. doi: 10.1016/j.ejca.2019.11.014. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

