Effect of the Synthetic Parameters over ZnO in the CO2 Photoreduction
- PMID: 37375353
- PMCID: PMC10301045
- DOI: 10.3390/molecules28124798
Effect of the Synthetic Parameters over ZnO in the CO2 Photoreduction
Abstract
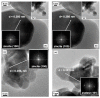
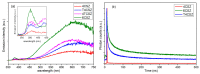
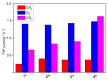
Zinc oxide (ZnO) is an attractive semiconductor material for photocatalytic applications, owing to its opto-electronic properties. Its performances are, however, strongly affected by the surface and opto-electronic properties (i.e., surface composition, facets and defects), in turn related to the synthesis conditions. The knowledge on how these properties can be tuned and how they are reflected on the photocatalytic performances (activity and stability) is thus essential to achieve an active and stable material. In this work, we studied how the annealing temperature (400 °C vs. 600 °C) and the addition of a promoter (titanium dioxide, TiO2) can affect the physico-chemical properties of ZnO materials, in particular surface and opto-electronic ones, prepared through a wet-chemistry method. Then, we explored the application of ZnO as a photocatalyst in CO2 photoreduction, an appealing light-to-fuel conversion process, with the aim to understand how the above-mentioned properties can affect the photocatalytic activity and selectivity. We eventually assessed the capability of ZnO to act as both photocatalyst and CO2 adsorber, thus allowing the exploitation of diluted CO2 sources as a carbon source.
Keywords: carbon dioxide adsorber; carbon dioxide photoreduction; opto-electronic properties; surface properties; zinc oxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Janotti A., Van de Walle C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009;72:126501. doi: 10.1088/0034-4885/72/12/126501. - DOI
-
- Ozgür Ü., Alivov Y.I., Liu C., Teke A., Reshchikov M.A., Doğan S., Avrutin V., Cho S.-J., Morkoç H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005;98:041301. doi: 10.1063/1.1992666. - DOI
-
- Pearton S.J., Ren F. ZnO-based materials for light emitting diodes. Curr. Opin. Chem. Eng. 2014;3:51–55. doi: 10.1016/j.coche.2013.11.002. - DOI
-
- Petti L., Münzenrieder N., Vogt C., Faber H., Büthe L., Cantarella G., Bottacchi F., Anthopoulos T.D., Tröster G. Metal oxide semiconductor thin-film transistors for flexible electronics. Appl. Phys. Rev. 2016;3:021303. doi: 10.1063/1.4953034. - DOI
-
- Vittal R., Ho K.-C. Zinc oxide based dye-sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2017;70:920–935. doi: 10.1016/j.rser.2016.11.273. - DOI

