Design, Synthesis, Molecular Modeling, and Biological Evaluation of Novel Pyrimidine Derivatives as Potential Calcium Channel Blockers
- PMID: 37375424
- PMCID: PMC10303112
- DOI: 10.3390/molecules28124869
Design, Synthesis, Molecular Modeling, and Biological Evaluation of Novel Pyrimidine Derivatives as Potential Calcium Channel Blockers
Abstract
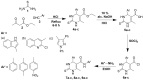
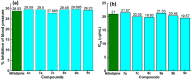
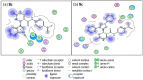
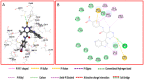
Pyrimidines play an important role in modern medical fields. They have a wide spectrum of biological activities such as antimicrobial, anticancer, anti-allergic, anti-leishmanial, antioxidant agents and others. Moreover, in recent years, 3,4-dihydropyrimidin-2(1H)ones have attracted researchers to synthesize them via Biginelli reaction and evaluate their antihypertensive activities as bioisosters of Nifedipine, which is a famous calcium channel blocker. Our new target compounds were prepared through one-pot reaction of thiourea 1, ethyl acetoacetate 2 and/or 1H-indole-2-carbaldehyde, 2-chloroquinoline-3-carbaldehyde, 1,3-diphenyl-1H-pyrazole-4-carbaldehyde, 3a-c in acid medium (HCl) yielding pyrimidines 4a-c, which in turn were hydrolyzed to carboxylic acid derivatives 5a-c which were chlorinated by SOCl2 to give acyl chlorides 6a-c. Finally, the latter were reacted with some selected aromatic amines, namely, aniline, p-toluidine and p-nitroaniline, producing amides 7a-c, 8a-c, and 9a-c. The purity of the prepared compounds was examined via TLC monitoring, and structures were confirmed by different spectroscopic techniques such as IR, 1HNMR, 13CNMR, and mass spectroscopy. The in vivo evaluation of the antihypertensive activity revealed that compounds 4c, 7a, 7c, 8c, 9b and 9c had comparable antihypertensive properties with Nifedipine. On the other hand, the in vitro calcium channel blocking activity was evaluated by IC50 measurement and results revealed that compounds 4c, 7a, 7b, 7c, 8c, 9a, 9b, and 9c had comparable calcium channel blocking activity with the reference Nifedipine. Based on the aforementioned biological results, we selected compounds 8c and 9c to be docked onto Ryanodine and dihydropyridine receptors. Furthermore, we developed a structure-activity relationship. The designed compounds in this study show promising activity profiles in reducing blood pressure and as calcium channel blockers, and could be considered as new potential antihypertensive and/or antianginal agents.
Keywords: 3,4-dihydropyrimidin-2(1H)ones; antihypertensive; calcium channel blocking; nifedipine isosters.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Matchar D.B., McCrory D.C., Orlando L.A., Patel M.R., Patel U.D., Patwardhan M.B., Powers B., Samsa G.P., Gray R.N. Systematic review: Comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann. Intern. Med. 2008;148:16–29. doi: 10.7326/0003-4819-148-1-200801010-00189. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

