Impact of Intravenous Iron Substitution on Serum Phosphate Levels and Bone Turnover Markers-An Open-Label Pilot Study
- PMID: 37375595
- PMCID: PMC10304306
- DOI: 10.3390/nu15122693
Impact of Intravenous Iron Substitution on Serum Phosphate Levels and Bone Turnover Markers-An Open-Label Pilot Study
Abstract
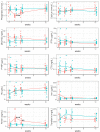
The association between intravenous iron substitution therapy and hypophosphatemia was previously reported in patients with iron deficiency anemia. However, the extent of hypophosphatemia is thought to depend on the type of iron supplementation. We hypothesized that the intravenous application of ferric carboxymaltose and iron sucrose leads to a different longitudinal adaptation in serum phosphate levels. In this open-label pilot study, a total of 20 patients with inflammatory bowel diseases or iron deficiency anemia were randomly assigned to one of two study groups (group 1: ferric carboxymaltose, n = 10; group 2: iron sucrose, n = 10). Serum values were controlled before iron substitution therapy, as well as 2, 4, and 12 weeks after the last drug administration. The primary objective of the study was the longitudinal evaluation of serum phosphate levels after iron substitution therapy with ferric carboxymaltose and iron sucrose. The secondary objective was the longitudinal investigation of calcium, 25-hydroxyvitamin D (25(OH)D), intact parathyroid hormone, procollagen type 1 amino-terminal propeptide (P1NP), beta-CrossLaps (CTX), hemoglobin (Hb), iron, ferritin, and transferrin saturation levels. Two weeks after drug administration, phosphate levels were significantly lower (p < 0.001) in group 1 and ferritin levels were significantly higher (p < 0.001) in group 1. Phosphate levels (0.8-1.45 mmol/L) were below the therapeutic threshold and ferritin levels (10-200 ng/mL for women and 30-300 ng/mL for men) were above the therapeutic threshold in group 1. P1NP (15-59 µg/L) and CTX (<0.57 ng/mL) levels were above the therapeutic threshold in group 2. Four weeks after drug administration, significant differences were still observed between both study groups for phosphate (p = 0.043) and ferritin (p = 0.0009). All serum values except for Hb were within the therapeutic thresholds. Twelve weeks after drug administration, no differences were observed in all serum values between both study groups. Hb values were within the therapeutic threshold in both study groups. Serum 25(OH)D levels did not differ between both study groups throughout the whole study period and remained within the therapeutic threshold.
Keywords: 25-hydroxyvitamin D (25(OH)D); beta-CrossLaps (CTX); bone turnover markers; ferric carboxymaltose; hypophosphatemia; iron substitution therapy; iron sucrose; phosphate; procollagen type 1 amino-terminal propeptide (P1NP).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Shimizu Y., Tada Y., Yamauchi M., Okamoto T., Suzuki H., Ito N., Fukumoto S., Sugimoto T., Fujita T. Hypophosphatemia induced by intravenous administration of saccharated ferric oxide: Another form of FGF23-related hypophosphatemia. Bone. 2009;45:814–816. doi: 10.1016/j.bone.2009.06.017. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

