Phase II, Double-Blinded, Randomized, Placebo-Controlled Clinical Trial Investigating the Efficacy of Mebendazole in the Management of Symptomatic COVID-19 Patients
- PMID: 37375747
- PMCID: PMC10300804
- DOI: 10.3390/ph16060799
Phase II, Double-Blinded, Randomized, Placebo-Controlled Clinical Trial Investigating the Efficacy of Mebendazole in the Management of Symptomatic COVID-19 Patients
Abstract
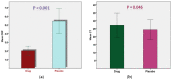
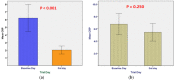
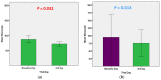
The outbreak of the COVID-19 pandemic has spread throughout the world, affecting almost all nations and territories. The current double-blind, randomized, placebo-controlled, phase II clinical trial sought to evaluate the clinical efficacy and safety of mebendazole as an adjuvant therapy for outpatients with COVID-19. The patients were recruited and divided into two groups: a Mebendazole-treated group and placebo group. The mebendazole and placebo groups were matched for age, sex, and complete blood count (CBC) with differential and liver and kidney function tests at baseline. On the third day, the C-reactive protein (CRP) levels were lower (2.03 ± 1.45 vs. 5.45 ± 3.95, p < 0.001) and the cycle threshold (CT) levels were higher (27.21 ± 3.81 vs. 24.40 ± 3.09, p = 0.046) significantly in the mebendazole group than in the placebo group on the third day. Furthermore, CRP decreased and CT dramatically increased on day three compared to the baseline day in the mebendazole group (p < 0.001 and p = 0.008, respectively). There was a significant inverse correlation between lymphocytes and CT levels in the mebendazole group (r = -0.491, p = 0.039) but not in the placebo group (r = 0.051, p = 0.888). Mebendazole therapy increased innate immunity and returned inflammation to normal levels in COVID-19 outpatients faster than it did in the placebo group in this clinical trial. Our findings add to the growing body of research on the clinical and microbiological benefits of repurposing antiparasitic therapy, specifically mebendazole, for SARS-CoV-2 infection and other viral infections.
Keywords: COVID-19 outpatients; mebendazole; placebo-controlled clinical trial; repurposing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Worldometer COVID-19 Coronavirus Pandemic. [(accessed on 10 May 2022)]. Available online: https://www.worldometers.info/coronavirus/
-
- Worldometer CWG.Coronavirus Worldwide Graphs. [(accessed on 25 May 2022)]. Available online: https://www.worldometers.info/coronavirus/worldwide-graphs/#total-deaths.
-
- World Health Organization Jordan: WHO Coronavirus Disease (COVID-19) Dashboard with Vaccination Data. [(accessed on 11 May 2022)]. Available online: https://covid19.who.int/region/emro/country/jo.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

