Live Cell Imaging by Förster Resonance Energy Transfer Fluorescence to Study Trafficking of PLGA Nanoparticles and the Release of a Loaded Peptide in Dendritic Cells
- PMID: 37375766
- PMCID: PMC10304224
- DOI: 10.3390/ph16060818
Live Cell Imaging by Förster Resonance Energy Transfer Fluorescence to Study Trafficking of PLGA Nanoparticles and the Release of a Loaded Peptide in Dendritic Cells
Abstract
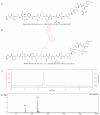
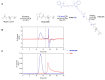
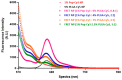
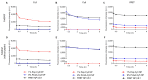
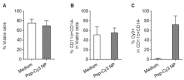
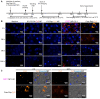
Our previous study demonstrated that a selected β-lactoglobulin-derived peptide (BLG-Pep) loaded in poly(lactic-co-glycolic acid) (PLGA) nanoparticles protected mice against cow's milk allergy development. However, the mechanism(s) responsible for the interaction of the peptide-loaded PLGA nanoparticles with dendritic cells (DCs) and their intracellular fate was/were elusive. Förster resonance energy transfer (FRET), a distance-dependent non-radioactive energy transfer process mediated from a donor to an acceptor fluorochrome, was used to investigate these processes. The ratio of the donor (Cyanine-3)-conjugated peptide and acceptor (Cyanine-5) labeled PLGA nanocarrier was fine-tuned for optimal (87%) FRET efficiency. The colloidal stability and FRET emission of prepared NPs were maintained upon 144 h incubation in PBS buffer and 6 h incubation in biorelevant simulated gastric fluid at 37 °C. A total of 73% of Pep-Cy3 NP was internalized by DCs as quantified using flow cytometry and confirmed using confocal fluorescence microscopy. By real-time monitoring of the change in the FRET signal of the internalized peptide-loaded nanoparticles, we observed prolonged retention (for 96 h) of the nanoparticles-encapsulated peptide as compared to 24 h retention of the free peptide in the DCs. The prolonged retention and intracellular antigen release of the BLG-Pep loaded in PLGA nanoparticles in murine DCs might facilitate antigen-specific tolerance induction.
Keywords: Förster resonance energy transfer; cyanine-3; cyanine-5; dendritic cells; peptide delivery; poly(lactic-co-glycolic acid) nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Oral pretreatment with β-lactoglobulin derived peptide and CpG co-encapsulated in PLGA nanoparticles prior to sensitizations attenuates cow's milk allergy development in mice.Front Immunol. 2023 Jan 6;13:1053107. doi: 10.3389/fimmu.2022.1053107. eCollection 2022. Front Immunol. 2023. PMID: 36703973 Free PMC article.
-
PLGA nanoparticles loaded with beta-lactoglobulin-derived peptides modulate mucosal immunity and may facilitate cow's milk allergy prevention.Eur J Pharmacol. 2018 Jan 5;818:211-220. doi: 10.1016/j.ejphar.2017.10.051. Epub 2017 Oct 25. Eur J Pharmacol. 2018. PMID: 29079360
-
Inhibition of cow's milk allergy development in mice by oral delivery of β-lactoglobulin-derived peptides loaded PLGA nanoparticles is associated with systemic whey-specific immune silencing.Clin Exp Allergy. 2022 Jan;52(1):137-148. doi: 10.1111/cea.13967. Epub 2021 Jul 1. Clin Exp Allergy. 2022. PMID: 34145667 Free PMC article.
-
Förster Resonance Energy Transfer-Based Stability Assessment of PLGA Nanoparticles in Vitro and in Vivo.ACS Appl Bio Mater. 2019 Mar 18;2(3):1131-1140. doi: 10.1021/acsabm.8b00754. Epub 2019 Feb 5. ACS Appl Bio Mater. 2019. PMID: 30906926 Free PMC article.
-
Uptake and trafficking of different sized PLGA nanoparticles by dendritic cells in imiquimod-induced psoriasis-like mice model.Acta Pharm Sin B. 2021 Apr;11(4):1047-1055. doi: 10.1016/j.apsb.2020.11.008. Epub 2020 Nov 20. Acta Pharm Sin B. 2021. PMID: 33996416 Free PMC article.
Cited by
-
Recent Advances in Peptide-Loaded PLGA Nanocarriers for Drug Delivery and Regenerative Medicine.Pharmaceuticals (Basel). 2025 Jan 18;18(1):127. doi: 10.3390/ph18010127. Pharmaceuticals (Basel). 2025. PMID: 39861188 Free PMC article. Review.
References
-
- van Esch B.C., Schouten B., de Kivit S., Hofman G.A., Knippels L.M., Willemsen L.E., Garssen J. Oral tolerance induction by partially hydrolyzed whey protein in mice is associated with enhanced numbers of Foxp3+ regulatory T-cells in the mesenteric lymph nodes. Pediatr. Allergy Immunol. 2011;22:820–826. doi: 10.1111/j.1399-3038.2011.01205.x. - DOI - PubMed
-
- Meulenbroek L.A., van Esch B.C., Hofman G.A., den Hartog Jager C.F., Nauta A.J., Willemsen L.E., Bruijnzeel-Koomen C.A., Garssen J., van Hoffen E., Knippels L.M. Oral treatment with beta-lactoglobulin peptides prevents clinical symptoms in a mouse model for cow’s milk allergy. Pediatr. Allergy Immunol. 2013;24:656–664. doi: 10.1111/pai.12120. - DOI - PubMed
-
- Liu M., Thijssen S., van Nostrum C.F., Hennink W.E., Garssen J., Willemsen L.E.M. Inhibition of cow’s milk allergy development in mice by oral delivery of beta-lactoglobulin-derived peptides loaded PLGA nanoparticles is associated with systemic whey-specific immune silencing. Clin. Exp. Allergy. 2022;52:137–148. doi: 10.1111/cea.13967. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

