Inhibition of TRPA1, Endoplasmic Reticulum Stress, Human Airway Epithelial Cell Damage, and Ectopic MUC5AC Expression by Vasaka (Adhatoda vasica; Malabar Nut) Tea
- PMID: 37375837
- PMCID: PMC10303053
- DOI: 10.3390/ph16060890
Inhibition of TRPA1, Endoplasmic Reticulum Stress, Human Airway Epithelial Cell Damage, and Ectopic MUC5AC Expression by Vasaka (Adhatoda vasica; Malabar Nut) Tea
Abstract
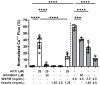
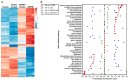
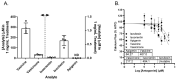
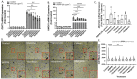
This study tested whether a medicinal plant, Vasaka, typically consumed as a tea to treat respiratory malaise, could protect airway epithelial cells (AECs) from wood smoke particle-induced damage and prevent pathological mucus production. Wood/biomass smoke is a pneumotoxic air pollutant. Mucus normally protects the airways, but excessive production can obstruct airflow and cause respiratory distress. Vasaka tea pre- and co-treatment dose-dependently inhibited mucin 5AC (MUC5AC) mRNA induction by AECs treated with wood smoke particles. This correlated with transient receptor potential ankyrin-1 (TRPA1) inhibition, an attenuation of endoplasmic reticulum (ER) stress, and AEC damage/death. Induction of mRNA for anterior gradient 2, an ER chaperone/disulfide isomerase required for MUC5AC production, and TRP vanilloid-3, a gene that suppresses ER stress and wood smoke particle-induced cell death, was also attenuated. Variable inhibition of TRPA1, ER stress, and MUC5AC mRNA induction was observed using selected chemicals identified in Vasaka tea including vasicine, vasicinone, apigenin, vitexin, isovitexin, isoorientin, 9-oxoODE, and 9,10-EpOME. Apigenin and 9,10-EpOME were the most cytoprotective and mucosuppressive. Cytochrome P450 1A1 (CYP1A1) mRNA was also induced by Vasaka tea and wood smoke particles. Inhibition of CYP1A1 enhanced ER stress and MUC5AC mRNA expression, suggesting a possible role in producing protective oxylipins in stressed cells. The results provide mechanistic insights and support for the purported benefits of Vasaka tea in treating lung inflammatory conditions, raising the possibility of further development as a preventative and/or restorative therapy.
Keywords: MUC5AC; TRPA1; Vasaka; airway epithelium; biomass smoke; lung injury; mucus hypersecretion; wood smoke.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures






References
-
- Rachana B.S., Mamta P., Priyanka K.M., Sonam S. Review & Future Perspectives of Using Vasicine, and Related Compounds. Indo-Glob. J. Pharm. Sci. 2011;1:85–98.
-
- Tuazon J.A., Kilburg-Basnyat B., Oldfield L.M., Wiscovitch-Russo R., Dunigan-Russell K., Fedulov A.V., Oestreich K.J., Gowdy K.M. Emerging Insights into the Impact of Air Pollution on Immune-Mediated Asthma Pathogenesis. Curr. Allergy Asthma Rep. 2022;22:77–92. doi: 10.1007/s11882-022-01034-1. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

