Tracking Permeation of Dimethyl Sulfoxide (DMSO) in Mentha × piperita Shoot Tips Using Coherent Raman Microscopy
- PMID: 37375873
- PMCID: PMC10304857
- DOI: 10.3390/plants12122247
Tracking Permeation of Dimethyl Sulfoxide (DMSO) in Mentha × piperita Shoot Tips Using Coherent Raman Microscopy
Abstract
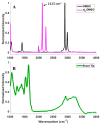
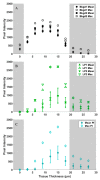
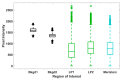
Cryopreservation has emerged as a low-maintenance, cost-effective solution for the long-term preservation of vegetatively propagated crops. Shoot tip cryopreservation often makes use of vitrification methods that employ highly concentrated mixtures of cryoprotecting agents; however, little is understood as to how these cryoprotecting agents protect cells and tissues from freezing. In this study, we use coherent anti-Stokes Raman scattering microscopy to directly visualize where dimethyl sulfoxide (DMSO) localizes within Mentha × piperita shoot tips. We find that DMSO fully penetrates the shoot tip tissue within 10 min of exposure. Variations in signal intensities across images suggest that DMSO may interact with cellular components, leading to its accumulation in specific regions.
Keywords: CARS microscopy; Raman; brightfield microscopy; cryoprotectant distribution; peppermint; plant cryopreservation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Engelmann F. Use of Biotechnologies for the Conservation of Plant Biodiversity. Vitro Cell. Dev. Biol. Plant. 2011;47:5–16. doi: 10.1007/s11627-010-9327-2. - DOI
-
- Jenderek M.M., Reed B.M. Cryopreserved Storage of Clonal Germplasm in the USDA National Plant Germplasm System. Vitro Cell. Dev. Biol. Plant. 2017;53:299–308. doi: 10.1007/s11627-017-9828-3. - DOI
-
- Engelmann F. Plant Cryopreservation: Progress and Prospects. Vitro Cell. Dev. Biol. Plant. 2004;40:427–433. doi: 10.1079/IVP2004541. - DOI
-
- Bettoni J.C., Bonnart R., Volk G.M. Challenges in Implementing Plant Shoot Tip Cryopreservation Technologies. Plant Cell Tissue Organ Cult. 2021;144:21–34. doi: 10.1007/s11240-020-01846-x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

