Recent Advances in Nanoformulations for Quercetin Delivery
- PMID: 37376104
- PMCID: PMC10302355
- DOI: 10.3390/pharmaceutics15061656
Recent Advances in Nanoformulations for Quercetin Delivery
Abstract
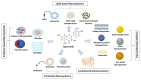
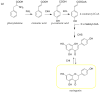
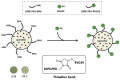
Quercetin (QUE) is a flavonol that has recently received great attention from the research community due to its important pharmacological properties. However, QUE's low solubility and extended first-pass metabolism limit its oral administration. This review aims to present the potential of various nanoformulations in the development of QUE dosage forms for bioavailability enhancement. Advanced drug delivery nanosystems can be used for more efficient encapsulation, targeting, and controlled release of QUE. An overview of the primary nanosystem categories, formulation processes, and characterization techniques are described. In particular, lipid-based nanocarriers, such as liposomes, nanostructured-lipid carries, and solid-lipid nanoparticles, are widely used to improve QUE's oral absorption and targeting, increase its antioxidant activity, and ensure sustained release. Moreover, polymer-based nanocarriers exhibit unique properties for the improvement of the Absorption, Distribution, Metabolism, Excretion, and Toxicology (ADME(T)) profile. Namely, micelles and hydrogels composed of natural or synthetic polymers have been applied in QUE formulations. Furthermore, cyclodextrin, niosomes, and nanoemulsions are proposed as formulation alternatives for administration via different routes. This comprehensive review provides insight into the role of advanced drug delivery nanosystems for the formulation and delivery of QUE.
Keywords: anti-inflammatory; antioxidant; drug-delivery systems; nanoformulations; quercetin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Zhang Y., Guan R., Huang H. Anti-Allergic Effects of Quercetin and Quercetin Liposomes in RBL-2H3 Cells. Endocr. Metab. Immune Disord. Drug Targets. 2022;23:692–701. - PubMed
-
- Diamantis D., Ramesova S., Chatzigiannis C., Degano I., Gerogianni P., Karadima C., Perikleous S., Rekkas D., Gerothanassis I., Galaris D., et al. Exploring the oxidation and iron binding profile of a cyclodextrin encapsulated quercetin complex unveiled a controlled complex dissociation through a chemical stimulus. BBA-Gen. Subj. 2018;1862:1913–1924. doi: 10.1016/j.bbagen.2018.06.006. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

