Visualization and Estimation of Nasal Spray Delivery to Olfactory Mucosa in an Image-Based Transparent Nasal Model
- PMID: 37376105
- PMCID: PMC10303528
- DOI: 10.3390/pharmaceutics15061657
Visualization and Estimation of Nasal Spray Delivery to Olfactory Mucosa in an Image-Based Transparent Nasal Model
Abstract
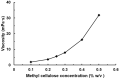
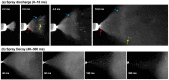
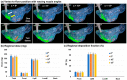
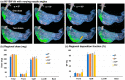
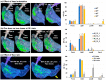
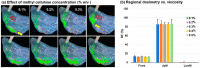
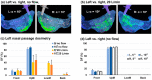
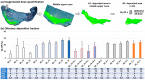
Background: Nose-to-brain (N2B) drug delivery offers unique advantages over intravenous methods; however, the delivery efficiency to the olfactory region using conventional nasal devices and protocols is low. This study proposes a new strategy to effectively deliver high doses to the olfactory region while minimizing dose variability and drug losses in other regions of the nasal cavity. Materials and Methods: The effects of delivery variables on the dosimetry of nasal sprays were systematically evaluated in a 3D-printed anatomical model that was generated from a magnetic resonance image of the nasal airway. The nasal model comprised four parts for regional dose quantification. A transparent nasal cast and fluorescent imaging were used for visualization, enabling detailed examination of the transient liquid film translocation, real-time feedback on input effect, and prompt adjustment to delivery variables, which included the head position, nozzle angle, applied dose, inhalation flow, and solution viscosity. Results: The results showed that the conventional vertex-to-floor head position was not optimal for olfactory delivery. Instead, a head position tilting 45-60° backward from the supine position gave a higher olfactory deposition and lower variability. A two-dose application (250 mg) was necessary to mobilize the liquid film that often accumulated in the front nose following the first dose administration. The presence of an inhalation flow reduced the olfactory deposition and redistributed the sprays to the middle meatus. The recommended olfactory delivery variables include a head position ranging 45-60°, a nozzle angle ranging 5-10°, two doses, and no inhalation flow. With these variables, an olfactory deposition fraction of 22.7 ± 3.7% was achieved in this study, with insignificant discrepancies in olfactory delivery between the right and left nasal passages. Conclusions: It is feasible to deliver clinically significant doses of nasal sprays to the olfactory region by leveraging an optimized combination of delivery variables.
Keywords: head position; intranasal drug delivery; liquid film translocation; nasal spray; nose-to-brain (N2B); soft-mist inhaler; vertex-to-floor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Liquid Film Translocation Significantly Enhances Nasal Spray Delivery to Olfactory Region: A Numerical Simulation Study.Pharmaceutics. 2021 Jun 18;13(6):903. doi: 10.3390/pharmaceutics13060903. Pharmaceutics. 2021. PMID: 34207109 Free PMC article.
-
A Supine Position and Dual-Dose Applications Enhance Spray Dosing to the Posterior Nose: Paving the Way for Mucosal Immunization.Pharmaceutics. 2023 Jan 20;15(2):359. doi: 10.3390/pharmaceutics15020359. Pharmaceutics. 2023. PMID: 36839681 Free PMC article.
-
Delivery of Agarose-aided Sprays to the Posterior Nose for Mucosa Immunization and Short-term Protection against Infectious Respiratory Diseases.Curr Pharm Biotechnol. 2024;25(6):787-798. doi: 10.2174/1389201024666230801142913. Curr Pharm Biotechnol. 2024. PMID: 37533243
-
Bi-directional nasal drug delivery systems: A scoping review of nasal particle deposition patterns and clinical application.Laryngoscope Investig Otolaryngol. 2023 Nov 22;8(6):1484-1499. doi: 10.1002/lio2.1190. eCollection 2023 Dec. Laryngoscope Investig Otolaryngol. 2023. PMID: 38130248 Free PMC article.
-
Positional Installation of Intranasal Corticosteroids in the Treatment of Chronic Rhinosinusitis: A Systematic Review of the Literature.Ear Nose Throat J. 2021 Jun;100(5):302-308. doi: 10.1177/0145561320961004. Epub 2020 Sep 24. Ear Nose Throat J. 2021. PMID: 32970499
Cited by
-
Comparative study of nasal cavity drug delivery efficiency with different nozzles in a 3D printed model.PeerJ. 2024 Apr 10;12:e17227. doi: 10.7717/peerj.17227. eCollection 2024. PeerJ. 2024. PMID: 38618567 Free PMC article.
-
Assessing Nasal Epithelial Dynamics: Impact of the Natural Nasal Cycle on Intranasal Spray Deposition.Pharmaceuticals (Basel). 2024 Jan 6;17(1):73. doi: 10.3390/ph17010073. Pharmaceuticals (Basel). 2024. PMID: 38256906 Free PMC article.
-
Optimized gravity-driven intranasal drop administration delivers significant doses to the ostiomeatal complex and maxillary sinus.Drug Deliv Transl Res. 2024 Jul;14(7):1839-1859. doi: 10.1007/s13346-023-01488-4. Epub 2023 Dec 4. Drug Deliv Transl Res. 2024. PMID: 38044376
-
Effects of Nozzle Retraction Elimination on Spray Distribution in Middle-Posterior Turbinate Regions: A Comparative Study.Pharmaceutics. 2024 May 19;16(5):683. doi: 10.3390/pharmaceutics16050683. Pharmaceutics. 2024. PMID: 38794345 Free PMC article.
-
Advances in Aerosol Formulation for Targeted Delivery of Therapeutic Agents from Nose to Brain.Curr Drug Deliv. 2025;22(6):678-693. doi: 10.2174/0115672018285350240227073607. Curr Drug Deliv. 2025. PMID: 38445697 Review.
References
-
- Frey W.H., Liu J., Chen X., Thorne R.G., Fawcett J.R., Ala T.A., Rahman Y.-E. Delivery of 125I-NGF to the Brain via the Olfactory Route. Drug Deliv. 1997;4:87–92. doi: 10.3109/10717549709051878. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

