Novel Bioequivalent Tablet of Solifenacin Succinate Prepared Using Direct Compression Technique for Improved Chemical Stability
- PMID: 37376171
- PMCID: PMC10301382
- DOI: 10.3390/pharmaceutics15061723
Novel Bioequivalent Tablet of Solifenacin Succinate Prepared Using Direct Compression Technique for Improved Chemical Stability
Abstract
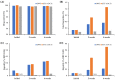
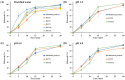
We designed a bioequivalent tablet form of solifenacin succinate (SOL) with an improved storage stability using a direct compression (DC) technique. An optimal direct compressed tablet (DCT) containing an active substance (10 mg), lactose monohydrate, and silicified microcrystalline cellulose as diluents, crospovidone as a disintegrant, and hydrophilic fumed silica as an anti-coning agent was constructed by evaluating the drug content uniformity, mechanical properties, and in vitro dissolution. The physicochemical and mechanical properties of the DCT were as follows: drug content 100.1 ± 0.7%, disintegration time of 6.7 min, over 95% release within 30 min in dissolution media (pH 1.2, 4.0, 6.8, and distilled water), hardness > 107.8 N, and friability ~0.11%. The SOL-loaded tablet fabricated via DC showed an improved stability at 40 °C and RH 75%, exhibiting markedly reduced degradation products compared to those fabricated using ethanol or water-based wet granulation or a marketed product (Vesicare®, Astellas Pharma). Moreover, in a bioequivalence study in healthy subjects (n = 24), the optimized DCT offered a pharmacokinetic profile comparable to that of the marketed product, with no statistical differences in the pharmacokinetic parameters. The 90% CIs for the geometric mean ratios of the test to the reference formulation for the area under the curve and the maximum drug concentration in plasma were 0.98-1.05 and 0.98-1.07, respectively, and satisfied the FDA regulatory criteria for bioequivalence. Thus, we conclude that DCT is a beneficial oral dosage form of SOL with an improved chemical stability.
Keywords: bioequivalence; direct compression; pharmacokinetics; solid dosage form; solifenacin succinate; storage stability.
Conflict of interest statement
The authors declare no conflict of interest. The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures



References
-
- Franco I., Hoebeke P., Baka-Ostrowska M., Bolong D., Davies L.N., Dahler E., Snijder R., Stroosma O., Verheggen F., Newgreen D., et al. Long-Term Efficacy and Safety of Solifenacin in Pediatric Patients Aged 6 Months to 18 Years with Neurogenic Detrusor Overactivity: Results from Two Phase 3 Prospective Open-Label Studies. J. Pediatr. Urol. 2020;16 doi: 10.1016/j.jpurol.2019.12.012. - DOI - PubMed
-
- Mostafaei H., Salehi-Pourmehr H., Jilch S., Carlin G.L., Mori K., Quhal F., Pradere B., Grossmann N.C., Laukhtina E., Schuettfort V.M., et al. Choosing the Most Efficacious and Safe Oral Treatment for Idiopathic Overactive Bladder: A Systematic Review and Network Meta-Analysis. Eur. Urol. Focus. 2022;8:1072–1089. doi: 10.1016/j.euf.2021.08.011. - DOI - PubMed
-
- Niphade N.C., Jagtap K.M., Mali A.C., Solanki P.V., Jachak M.N., Mathad V.T. Efficient and Single Pot Process for the Preparation of Enantiomerically Pure Solifenacin Succinate, an Antimuscarinic Agent. Monatsh. Chem. 2011;142:1181–1186. doi: 10.1007/s00706-011-0610-7. - DOI
LinkOut - more resources
Full Text Sources

