Electrospun Fibrous Silica for Bone Tissue Engineering Applications
- PMID: 37376176
- PMCID: PMC10304373
- DOI: 10.3390/pharmaceutics15061728
Electrospun Fibrous Silica for Bone Tissue Engineering Applications
Abstract
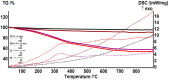
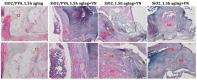
The production of highly porous and three-dimensional (3D) scaffolds with biomimicking abilities has gained extensive attention in recent years for tissue engineering (TE) applications. Considering the attractive and versatile biomedical functionality of silica (SiO2) nanomaterials, we propose herein the development and validation of SiO2-based 3D scaffolds for TE. This is the first report on the development of fibrous silica architectures, using tetraethyl orthosilicate (TEOS) and polyvinyl alcohol (PVA) during the self-assembly electrospinning (ES) processing (a layer of flat fibers must first be created in self-assembly electrospinning before fiber stacks can develop on the fiber mat). The compositional and microstructural characteristics of obtained fibrous materials were evaluated by complementary techniques, in both the pre-ES aging period and post-ES calcination. Then, in vivo evaluation confirmed their possible use as bioactive scaffolds in bone TE.
Keywords: electrospinning; fibrous silica; tissue regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Pramanik S., Kharche S., More N., Ranglani D., Singh G., Kapusetti G. Natural Biopolymers for Bone Tissue Engineering: A Brief Review. Eng. Regen. 2023;4:193–204. doi: 10.1016/j.engreg.2022.12.002. - DOI
-
- Beheshtizadeh N., Gharibshahian M., Pazhouhnia Z., Rostami M., Zangi A.R., Maleki R., Azar H.K., Zalouli V., Rajavand H., Farzin A., et al. Commercialization and regulation of regenerative medicine products: Promises, advances and challenges. Biomed. Pharmacother. 2022;153:113431. doi: 10.1016/j.biopha.2022.113431. - DOI - PubMed
-
- Jiang T., Carbone E.J., Lo K.W.-H., Laurencin C.T. Electrospinning of polymer nanofibers for tissue regeneration. Prog. Polym. Sci. 2015;46:1–24. doi: 10.1016/j.progpolymsci.2014.12.001. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

