Unraveling the Molecular and Cellular Pathogenesis of COVID-19-Associated Liver Injury
- PMID: 37376587
- PMCID: PMC10304875
- DOI: 10.3390/v15061287
Unraveling the Molecular and Cellular Pathogenesis of COVID-19-Associated Liver Injury
Abstract
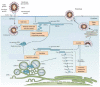
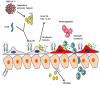
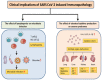
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus (SARS-CoV-2) continues to cause substantial morbidity and mortality. Most infections are mild; however, some patients experience severe and potentially fatal systemic inflammation, tissue damage, cytokine storm, and acute respiratory distress syndrome. Patients with chronic liver disease have been frequently affected, experiencing high morbidity and mortality. In addition, elevated liver enzymes may be a risk factor for disease progression, even in the absence of underlying liver disease. While the respiratory tract is a primary target of SARS-CoV-2, it has become evident that COVID-19 is a multisystemic infectious disease. The hepatobiliary system might be influenced during COVID-19 infection, ranging from a mild elevation of aminotransferases to the development of autoimmune hepatitis and secondary sclerosing cholangitis. Furthermore, the virus can promote existing chronic liver diseases to liver failure and activate the autoimmune liver disease. Whether the direct cytopathic effects of the virus, host reaction, hypoxia, drugs, vaccination, or all these risk factors cause liver injury has not been clarified to a large extent in COVID-19. This review article discussed the molecular and cellular mechanisms involved in the pathogenesis of SARS-CoV-2 virus-associated liver injury and highlighted the emerging role of liver sinusoidal epithelial cells (LSECs) in virus-related liver damage.
Keywords: COVID-19; SARS-CoV-2; autoimmune liver disease; chronic liver disease; liver injury; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

